Related Research Articles

Elias James Corey is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably.
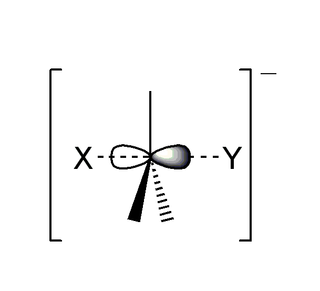
The SN2 reaction is a type of reaction mechanism that is common in organic chemistry. In this mechanism, one bond is broken and one bond is formed in a concerted way, i.e., in one step. The name SN2 refers to the Hughes-Ingold symbol of the mechanism: "SN" indicates that the reaction is a nucleophilic substitution, and "2" that it proceeds via a bi-molecular mechanism, which means both the reacting species are involved in the rate-determining step. The other major type of nucleophilic substitution is the SN1, but many other more specialized mechanisms describe substitution reactions.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 1988 that the "word synthon has now come to be used to mean synthetic building block rather than retrosynthetic fragmentation structures". It was noted in 1998 that the phrase did not feature very prominently in Corey's 1981 book The Logic of Chemical Synthesis, as it was not included in the index. Because synthons are charged, when placed into a synthesis a neutral form is found commercially instead of forming and using the potentially very unstable charged synthons.

Organoboron chemistry or organoborane chemistry is the chemistry of organoboron compounds or organoboranes, which are chemical compounds of boron and carbon that are organic derivatives of borane (BH3), for example trialkyl boranes..

The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation.

The Baeyer–Villiger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant. The reaction is named after Adolf von Baeyer and Victor Villiger who first reported the reaction in 1899.

The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid but most of the time either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.
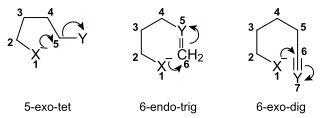
Baldwin's rules in organic chemistry are a series of guidelines outlining the relative favorabilities of ring closure reactions in alicyclic compounds. They were first proposed by Jack Baldwin in 1976.
The Nazarov cyclization reaction is a chemical reaction used in organic chemistry for the synthesis of cyclopentenones. The reaction is typically divided into classical and modern variants, depending on the reagents and substrates employed. It was originally discovered by Ivan Nikolaevich Nazarov (1906–1957) in 1941 while studying the rearrangements of allyl vinyl ketones.

Donna J. Nelson is an American chemist and professor of chemistry at the University of Oklahoma. Nelson specializes in organic chemistry, which she both researches and teaches. Nelson served as a science advisor to the AMC television show Breaking Bad. She was the 2016 President of the American Chemical Society (ACS) with her presidential activities focusing on and guided by communities in chemistry. Nelson's research focused on five primary topics, generally categorized in two areas, Scientific Research and America's Scientific Readiness. Within Scientific Research, Nelson's topics have been on mechanistic patterns in alkene addition reactions and on Single-Walled Carbon Nanotube (SWCNT) functionalization and analysis, yielding the first COSY NMR spectrum of covalently functionalized SWCNTs in solution. Under America's Scientific Readiness, she focuses on science education and impacting science by considering its communities; this includes classroom innovations and correcting organic chemistry textbook inaccuracies, on ethnic and gender diversity among highly ranked science departments of research universities, and on improving the image and presentation of science and scientists to the public.
Jeremy Keith Morris Sanders is a British chemist and Emeritus Professor in the Department of Chemistry at the University of Cambridge. He is also Editor-in-Chief of Royal Society Open Science. He is known for his contributions to many fields including NMR spectroscopy and supramolecular chemistry. He served as the Pro-Vice-Chancellor for Institutional Affairs at the University of Cambridge, 2011–2015.

Howard E. Zimmerman was a professor of chemistry at the University of Wisconsin–Madison. He was elected to the National Academy of Sciences in 1980 and the recipient of the 1986 American Institute of Chemists Chemical Pioneer Award.

Kenneth S. Suslick is the Marvin T. Schmidt Professor of Chemistry Emeritus at the University of Illinois at Urbana–Champaign. His area of focus is on the chemical and physical effects of ultrasound, sonochemistry, and sonoluminescence. In addition, he has worked in the fields of artificial and machine olfaction, electronic nose technology, chemical sensor arrays, and the use of colorimetric sensor arrays as an optoelectronic nose.
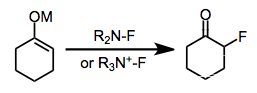
Electrophilic fluorination is the combination of a carbon-centered nucleophile with an electrophilic source of fluorine to afford organofluorine compounds. Although elemental fluorine and reagents incorporating an oxygen-fluorine bond can be used for this purpose, they have largely been replaced by reagents containing a nitrogen-fluorine bond.
In organic chemistry, α-halo ketones can be reduced with loss of the halogen atom to form enolates. The α-halo ketones are readily prepared from ketones by various ketone halogenation reactions, and the products are reactive intermediates that can be used for a variety of other chemical reactions.

Brian M. Stoltz is currently a professor of chemistry at the California Institute of Technology. The primary focus of his research is chemical synthesis with an emphasis on expanding the scope of allylic alkylation for the preparation of complex molecules possessing unique structural, biological, and physical properties. His research involves the total synthesis of natural products such as dragmacidin F and (–)-cyanthiwigin F, and development of synthetic reactions to access quaternary stereocenters. Specifically, he has focused on the allylic alkylation of enolates, developing an enantioselective variant in 2004.
In organic chemistry, the oxy-Cope rearrangement is a chemical reaction. It involves reorganization of the skeleton of certain unsaturated alcohols. It is a variation of the Cope rearrangement in which 1,5-dien-3-ols are converted to unsaturated carbonyl compounds by a mechanism typical for such a [3,3]-sigmatropic rearrangement.

Rick L. Danheiser is an American organic chemist and is the Arthur C. Cope Professor of Chemistry at the Massachusetts Institute of Technology and Chair of the MIT Faculty. His research involves the invention of new methods for the synthesis of complex organic compounds. Danheiser is known for the Danheiser annulation and Danheiser benzannulation reactions.

Stephen L. Craig is the William T. Miller Professor of Chemistry at Duke University. He is the director of the Center for Molecularly Optimized Networks, a NSF Center for Chemical Innovation.
References
- 1 2 "John Brauman wins 2002 National Medal of Science". Stanford Report. October 29, 2003. Retrieved June 17, 2013.
- ↑ The Allderdice. Seniors: John I. Brauman: Taylor Allderdice High School. 1955. p. 46.
- 1 2 3 4 5 "Biography". Stanford University. Archived from the original on April 1, 2013. Retrieved June 17, 2013.
- ↑ "National Medal of Science Recipient Details". National Science Foundation . Retrieved June 17, 2013.
- ↑ Wang, Linda (June 13, 2016). "John Brauman receives Parsons Award Stanford chemist honored for exceptional public service". Chemical & Engineering News. Retrieved 29 January 2018.