
The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation.

Cavicularin is a natural phenolic secondary metabolite isolated from the liverwort Cavicularia densa. This macrocycle is unusual because it was the first compound isolated from nature displaying optical activity solely due to the presence of planar chirality and axial chirality. The specific rotation for (+)-cavicularin is +168.2°. It is also a very strained molecule. The para-substituted phenol ring is bent about 15° out of planarity, adopting a somewhat boat-like geometry. This type of angle strain in aromatic compounds is normally reserved for synthetic cyclophanes.

Juncus effusus is a perennial herbaceous flowering plant species in the rush family Juncaceae, with the common names common rush or soft rush. In North America, the common name soft rush also refers to Juncus interior.
The Overman rearrangement is a chemical reaction that can be described as a Claisen rearrangement of allylic alcohols to give allylic trichloroacetamides through an imidate intermediate. The Overman rearrangement was discovered in 1974 by Larry Overman.

The Petasis reaction is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines.

Phenanthrenoids are chemical compounds formed with a phenanthrene backbone. These compounds occur naturally in plants, although they can also be synthesized.
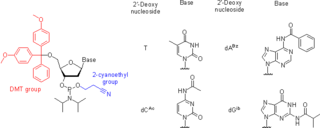
Nucleoside phosphoramidites are derivatives of natural or synthetic nucleosides. They are used to synthesize oligonucleotides, relatively short fragments of nucleic acid and their analogs. Nucleoside phosphoramidites were first introduced in 1981 by Beaucage and Caruthers. To avoid undesired side reactions, reactive hydroxy and exocyclic amino groups present in natural or synthetic nucleosides are appropriately protected. As long as a nucleoside analog contains at least one hydroxy group, the use of the appropriate protecting strategy allows one to convert that to the respective phosphoramidite and to incorporate the latter into synthetic nucleic acids. To be incorporated in the middle of an oligonucleotide chain using phosphoramidite strategy, the nucleoside analog must possess two hydroxy groups or, less often, a hydroxy group and another nucleophilic group (amino or mercapto). Examples include, but are not limited to, alternative nucleotides, LNA, morpholino, nucleosides modified at the 2'-position (OMe, protected NH2, F), nucleosides containing non-canonical bases (hypoxanthine and xanthine contained in natural nucleosides inosine and xanthosine, respectively, tricyclic bases such as G-clamp, etc.) or bases derivatized with a fluorescent group or a linker arm.
The Fukuyama coupling is a coupling reaction taking place between a thioester and an organozinc halide in the presence of a palladium catalyst. The reaction product is a ketone. This reaction was discovered by Tohru Fukuyama et al. in 1998.

Michael E. Jung is a Professor of Chemistry in the Department of Chemistry and Biochemistry at the University of California at Los Angeles.
The imine Diels–Alder reaction involves the transformation of all-carbon dienes and imine dienophiles into tetrahydropyridines.

Taxodone is a naturally occurring diterpenoid found in Taxodium distichum, Rosmarinus officinalis (rosemary), several salvia species and other plants, along with its oxidized rearrangement product, taxodione. Taxodone and taxodione exhibit anticancer, antibacterial, antioxidant, antifungal, insecticide, and antifeedant activities.

Allixin is a phytoalexin found in garlic bulbs. It was first isolated and characterized in 1989. When garlic is stored for long periods of time, it can form visible accumulations of crystalline allixin on its surface, particularly in areas where tissue has become necrotic. After 2 years of storage, the amount of allixin accumulated can approach 1% of the dry weight of the cloves. Since allixin has weak antimicrobial activity, these high concentrations are thought to be produced by the garlic bulb to protect itself from further damage from microorganisms.

Capnellene is a naturally occurring tricyclic hydrocarbon derived from Capnella imbricata, a species of soft coral found in Indonesia. Since the 1970s, capnellene has been targeted for synthesis by numerous investigators due to its stereochemistry, functionality, and the interesting geometry of the carbon skeleton. Many alcohol derivatives of capnellene have demonstrated potential as a chemotherapeutic agent with antibacterial, anti-inflammatory and anti-tumor properties.

Juncus roemerianus is a species of flowering plant in the rush family known by the common names black rush, needlerush, and black needlerush. It is native to North America, where its main distribution lies along the coastline of the southeastern United States, including the Gulf Coast. It occurs from New Jersey to Texas, with outlying populations in Connecticut, New York, Mexico, and certain Caribbean islands.
Trifluoromethylation in organic chemistry describes any organic reaction that introduces a trifluoromethyl group in an organic compound. Trifluoromethylated compounds are of some importance in pharmaceutical industry and agrochemicals. Several notable pharmaceutical compounds have a trifluoromethyl group incorporated: fluoxetine, mefloquine, Leflunomide, nulitamide, dutasteride, bicalutamide, aprepitant, celecoxib, fipronil, fluazinam, penthiopyrad, picoxystrobin, fluridone, norflurazon, sorafenib and triflurazin. A relevant agrochemical is trifluralin. The development of synthetic methods for adding trifluoromethyl groups to chemical compounds is actively pursued in academic research.

Trichloroacetonitrile is an organic compound with the formula CCl3CN. It is a colourless liquid, although commercial samples often are brownish. It is used commercially as a precursor to the fungicide etridiazole. It is prepared by dehydration of trichloroacetamide. As a bifunctional compound, trichloroacetonitrile can react at both the trichloromethyl and the nitrile group. The electron-withdrawing effect of the trichloromethyl group activates the nitrile group for nucleophilic additions. The high reactivity makes trichloroacetonitrile a versatile reagent, but also causes its susceptibility towards hydrolysis.
Fétizon oxidation is the oxidation of primary and secondary alcohols utilizing the compound silver(I) carbonate absorbed onto the surface of celite also known as Fétizon's reagent first employed by Marcel Fétizon in 1968. It is a mild reagent, suitable for both acid and base sensitive compounds. Its great reactivity with lactols makes the Fétizon oxidation a useful method to obtain lactones from a diol. The reaction is inhibited significantly by polar groups within the reaction system as well as steric hindrance of the α-hydrogen of the alcohol.
The Chan–Lam coupling reaction – also known as the Chan–Evans–Lam coupling is a cross-coupling reaction between an aryl boronic acid and an alcohol or an amine to form the corresponding secondary aryl amines or aryl ethers, respectively. The Chan–Lam coupling is catalyzed by copper complexes. It can be conducted in air at room temperature. The more popular Buchwald–Hartwig coupling relies on the use of palladium.
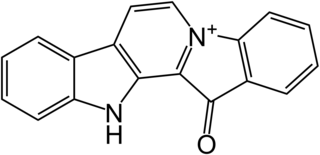
Fascaplysin is a marine alkaloid based on 12H-pyrido[1–2-a:3,4-b′]diindole ring system. It was first isolated as a red pigment from the marine sponge Fascaplysinopsis bergquist collected in the South Pacific near Fiji in 1988. Fascaplysin possesses a broad range of in vitro biological activities including analgesic, antimicrobial, antifungal, antiviral, antimalarial, anti-angiogenic, and antiproliferative activity against numerous cancer cell lines.
In organic chemistry, the Lombardo methylenation is a name reaction that allows for the methylenation of carbonyl compounds with the use of Lombardo's reagent, which is a mix of zinc, dibromomethane, and titanium tetrachloride.













