The actinide or actinoid series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part of the 6d transition series. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.

Otto Hahn was a German chemist who was a pioneer in the field of radiochemistry. He is referred to as the father of nuclear chemistry and discoverer of nuclear fission, the science behind nuclear reactors and nuclear weapons. Hahn and Lise Meitner discovered isotopes of the radioactive elements radium, thorium, protactinium and uranium. He also discovered the phenomena of atomic recoil and nuclear isomerism, and pioneered rubidium–strontium dating. In 1938, Hahn, Meitner and Fritz Strassmann discovered nuclear fission, for which Hahn alone was awarded the 1944 Nobel Prize in Chemistry.

Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in a process called Big Bang nucleosynthesis. After about 20 minutes, the universe had expanded and cooled to a point at which these high-energy collisions among nucleons ended, so only the fastest and simplest reactions occurred, leaving our universe containing hydrogen and helium. The rest is traces of other elements such as lithium and the hydrogen isotope deuterium. Nucleosynthesis in stars and their explosions later produced the variety of elements and isotopes that we have today, in a process called cosmic chemical evolution. The amounts of total mass in elements heavier than hydrogen and helium remains small, so that the universe still has approximately the same composition.

In nuclear astrophysics, the rapid neutron-capture process, also known as the r-process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron, the "heavy elements", with the other half produced by the p-process and s-process. The r-process usually synthesizes the most neutron-rich stable isotopes of each heavy element. The r-process can typically synthesize the heaviest four isotopes of every heavy element; of these, the heavier two are called r-only nuclei because they are created exclusively via the r-process. Abundance peaks for the r-process occur near mass numbers A = 82, A = 130 and A = 196.

In nuclear physics, a magic number is a number of nucleons such that they are arranged into complete shells within the atomic nucleus. As a result, atomic nuclei with a "magic" number of protons or neutrons are much more stable than other nuclei. The seven most widely recognized magic numbers as of 2019 are 2, 8, 20, 28, 50, 82, and 126.

Frank Harold Spedding was a Canadian-American chemist. He was a renowned expert on rare earth elements, and on extraction of metals from minerals. The uranium extraction process helped make it possible for the Manhattan Project to build the first atomic bombs.

The Oddo–Harkins rule holds that an element with an even atomic number is more abundant than the elements with immediately adjacent atomic numbers. For example, carbon, with atomic number 6, is more abundant than boron (5) and nitrogen (7). Generally, the relative abundance of an even atomic numbered element is roughly two orders of magnitude greater than the relative abundances of the immediately adjacent odd atomic numbered elements to either side. This pattern was first reported by Giuseppe Oddo in 1914 and William Draper Harkins in 1917. The Oddo–Harkins rule is true for all elements beginning with carbon produced by stellar nucleosynthesis but not true for the lightest elements below carbon produced by big bang nucleosynthesis and cosmic ray spallation.
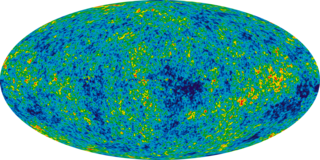
In physical cosmology, the Alpher–Bethe–Gamow paper, or αβγ paper, was created by Ralph Alpher, then a physics PhD student, his advisor George Gamow, and Hans Bethe. The work, which would become the subject of Alpher's PhD dissertation, argued that the Big Bang would create hydrogen, helium and heavier elements in the correct proportions to explain their abundance in the early universe. While the original theory neglected a number of processes important to the formation of heavy elements, subsequent developments showed that Big Bang nucleosynthesis is consistent with the observed constraints on all primordial elements.
BPS CS22892-0052 is an old population II star located at a distance of 4.7 kiloparsecs in the Milky Way's galactic halo. It belongs to a class of ultra-metal-poor stars, specifically the very rare subclass of neutron-capture (r-process) enhanced stars. It was discovered by Tim C. Beers and collaborators with the Curtis Schmidt telescope at the Cerro Tololo Inter-American Observatory in Chile. Extended high-resolution spectroscopic observations since around 1995 allowed observers to determine the abundances of 53 chemical elements in this star, as of December 2005 only second in number to the Sun.
BD+17°3248 is an old Population II star located at a distance of roughly 968 light-years in the Galactic Halo. It belongs to the class of ultra-metal-poor stars, especially the very rare subclass of neutron-capture (r-process) enhanced stars.

Nuclear astrophysics studies the origin of the chemical elements and isotopes, and the role of nuclear energy generation, in cosmic sources such as stars, supernovae, novae, and violent binary-star interactions. It is an interdisciplinary part of both nuclear physics and astrophysics, involving close collaboration among researchers in various subfields of each of these fields. This includes, notably, nuclear reactions and their rates as they occur in cosmic environments, and modeling of astrophysical objects where these nuclear reactions may occur, but also considerations of cosmic evolution of isotopic and elemental composition (often called chemical evolution). Constraints from observations involve multiple messengers, all across the electromagnetic spectrum (nuclear gamma-rays, X-rays, optical, and radio/sub-mm astronomy), as well as isotopic measurements of solar-system materials such as meteorites and their stardust inclusions, cosmic rays, material deposits on Earth and Moon). Nuclear physics experiments address stability (i.e., lifetimes and masses) for atomic nuclei well beyond the regime of stable nuclides into the realm of radioactive/unstable nuclei, almost to the limits of bound nuclei (the drip lines), and under high density (up to neutron star matter) and high temperature (plasma temperatures up to 109 K). Theories and simulations are essential parts herein, as cosmic nuclear reaction environments cannot be realized, but at best partially approximated by experiments.
Alastair G. W. Cameron was an American–Canadian astrophysicist and space scientist who was an eminent staff member of the Astronomy department of Harvard University. He was one of the founders of the field of nuclear astrophysics, advanced the theory that the Moon was created by the giant impact of a Mars-sized object with the early Earth, and was an early adopter of computer technology in astrophysics.
The B2FH paper was a landmark scientific paper on the origin of the chemical elements. The paper's title is Synthesis of the Elements in Stars, but it became known as B2FH from the initials of its authors: Margaret Burbidge, Geoffrey Burbidge, William A. Fowler, and Fred Hoyle. It was written from 1955 to 1956 at the University of Cambridge and Caltech, then published in Reviews of Modern Physics in 1957.

Isotopes are distinct nuclear species of the same chemical element. They have the same atomic number and position in the periodic table, but different nucleon numbers due to different numbers of neutrons in their nuclei. While all isotopes of a given element have similar chemical properties, they have different atomic masses and physical properties.
The Hans A. Bethe Prize, is presented annually by the American Physical Society. The prize honors outstanding work in theory, experiment or observation in the areas of astrophysics, nuclear physics, nuclear astrophysics, or closely related fields. The prize consists of $10,000 and a certificate citing the contributions made by the recipient.
Friedrich-Karl "Friedel“ Thielemann is a German-Swiss theoretical astrophysicist.

Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.

Nuclear fission was discovered in December 1938 by chemists Otto Hahn and Fritz Strassmann and physicists Lise Meitner and Otto Robert Frisch. Fission is a nuclear reaction or radioactive decay process in which the nucleus of an atom splits into two or more smaller, lighter nuclei and often other particles. The fission process often produces gamma rays and releases a very large amount of energy, even by the energetic standards of radioactive decay. Scientists already knew about alpha decay and beta decay, but fission assumed great importance because the discovery that a nuclear chain reaction was possible led to the development of nuclear power and nuclear weapons. Hahn was awarded the 1944 Nobel Prize in Chemistry for the discovery of nuclear fission.
James Wellington Truran Jr. was an American physicist, known for his research in nuclear astrophysics.









