In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer. The reactive center can be radical, anionic or cationic. Some cyclic monomers such as norbornene or cyclooctadiene can be polymerized to high molecular weight polymers by using metal catalysts. ROP is a versatile method for the synthesis of biopolymers.

Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word μᾶλον (malon) meaning 'apple'.

In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called bipolymers. Those obtained from three and four monomers are called terpolymers and quaterpolymers, respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material.

Dendrimers are highly ordered, branched polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The word dendron is also encountered frequently. A dendron usually contains a single chemically addressable group called the focal point or core. The difference between dendrons and dendrimers is illustrated in the top figure, but the terms are typically encountered interchangeably.

Robert Samuel Langer Jr. FREng is an American chemical engineer, scientist, entrepreneur, inventor and one of the twelve Institute Professors at the Massachusetts Institute of Technology.

Sebacoyl chloride (or sebacoyl dichloride) is a di-acyl chloride, with formula (CH2)8(COCl)2. A colorless oily liquid with a pungent odor, it is soluble in hydrocarbons and ethers. Sebacoyl chloride is corrosive; like all acyl chlorides, it hydrolyzes, evolving hydrogen chloride. It is less susceptible to hydrolysis though than shorter chain aliphatic acyl chlorides.

Polyphosphazenes include a wide range of hybrid inorganic-organic polymers with a number of different skeletal architectures with the backbone P-N-P-N-P-N-. In nearly all of these materials two organic side groups are attached to each phosphorus center. Linear polymers have the formula (N=PR1R2)n, where R1 and R2 are organic (see graphic). Other architectures are cyclolinear and cyclomatrix polymers in which small phosphazene rings are connected together by organic chain units. Other architectures are available, such as block copolymer, star, dendritic, or comb-type structures. More than 700 different polyphosphazenes are known, with different side groups (R) and different molecular architectures. Many of these polymers were first synthesized and studied in the research group of Harry R. Allcock.

Biodegradable polymers are a special class of polymer that breaks down after its intended purpose by bacterial decomposition process to result in natural byproducts such as gases (CO2, N2), water, biomass, and inorganic salts. These polymers are found both naturally and synthetically made, and largely consist of ester, amide, and ether functional groups. Their properties and breakdown mechanism are determined by their exact structure. These polymers are often synthesized by condensation reactions, ring opening polymerization, and metal catalysts. There are vast examples and applications of biodegradable polymers.
Polyelectrolytes are charged polymers capable of stabilizing colloidal emulsions through electrostatic interactions. Their effectiveness can be dependent on molecular weight, pH, solvent polarity, ionic strength, and the hydrophilic-lipophilic balance (HLB). Stabilized emulsions are useful in many industrial processes, including deflocculation, drug delivery, petroleum waste treatment, and food technology.
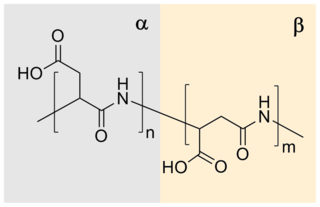
Polyaspartic acid (PASA) is a biodegradable, water-soluble condensation polymer based on the amino acid aspartic acid. It is a biodegradable replacement for water softeners and related applications. PASA can be chemically crosslinked with a wide variety of methods to yield PASA hydrogels. The resulting hydrogels are pH-sensitive such that under acidic conditions, they shrink, while the swelling capacity increases under alkaline conditions.

Poly(ethylene adipate) or PEA is an aliphatic polyester. It is most commonly synthesized from a polycondensation reaction between ethylene glycol and adipic acid. PEA has been studied as it is biodegradable through a variety of mechanisms and also fairly inexpensive compared to other polymers. Its lower molecular weight compared to many polymers aids in its biodegradability.

Matthew V. Tirrell is an American chemical engineer. In 2011 he became the Founding Pritzker Director and Dean of the Institute for Molecular Engineering (IME) at the University of Chicago, in addition to serving as senior scientist at Argonne National Laboratory. Tirrell's research specializes in the manipulation and measurement of polymer surface properties, polyelectrolyte complexation, and biomedical nanoparticles.
Polyorthoesters are polymers with the general structure –[–R–O–C(R1, OR2)–O–R3–]n– whereas the residue R2 can also be part of a heterocyclic ring with the residue R. Polyorthoesters are formed by transesterification of orthoesters with diols or by polyaddition between a diol and a diketene acetal, such as 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane.
Anne Hiltner was an American polymer scientist who founded the Center for Applied Polymer Research (CAPRI) and was later instrumental in the founding of the Center for Layer Polymeric Systems (CLiPS), a National Science Foundation Science and Technology Center at Case Western Reserve University. She served as Director of the Center for Layered Polymeric Systems from its founding in 2006 until her death in 2010.
Nanoparticle drug delivery systems are engineered technologies that use nanoparticles for the targeted delivery and controlled release of therapeutic agents. The modern form of a drug delivery system should minimize side-effects and reduce both dosage and dosage frequency. Recently, nanoparticles have aroused attention due to their potential application for effective drug delivery.
Theresa M. Reineke is an American chemist and Distinguished McKnight University Professor at the University of Minnesota. She designs sustainable, environmentally friendly polymer-based delivery systems for targeted therapeutics. She is the Associate Editor of ACS Macro Letters.

β-Butyrolactone is the intramolecular carboxylic acid ester (lactone) of the optically active 3-hydroxybutanoic acid. It is produced during chemical synthesis as a racemate. β-Butyrolactone is suitable as a monomer for the production of the biodegradable polyhydroxyalkanoate poly(3-hydroxybutyrate) (PHB). Polymerisation of racemic (RS)-β-butyrolactone provides (RS)-polyhydroxybutyric acid, which, however, is inferior in essential properties to the (R)-poly-3-hydroxybutyrate originating from natural sources.

Rodney Dewayne Priestley is an American chemical engineer and Professor at Princeton University. His research considers the phase transitions of polymers and their application in electronic devices and healthcare. In 2020 he was made the Princeton University Vice Dean of Innovation. He was named dean of The Graduate School effective June 1, 2022.

Dextran drug delivery systems involve the use of the natural glucose polymer dextran in applications as a prodrug, nanoparticle, microsphere, micelle, and hydrogel drug carrier in the field of targeted and controlled drug delivery. According to several in vitro and animal research studies, dextran carriers reduce off-site toxicity and improve local drug concentration at the target tissue site. This technology has significant implications as a potential strategy for delivering therapeutics to treat cancer, cardiovascular diseases, pulmonary diseases, bone diseases, liver diseases, colonic diseases, infections, and HIV.
Pullulan bioconjugates are systems that use pullulan as a scaffold to attach biological materials to, such as drugs. These systems can be used to enhance the delivery of drugs to specific environments or the mechanism of delivery. These systems can be used in order to deliver drugs in response to stimuli, create a more controlled and sustained release, and provide a more targeted delivery of certain drugs.














