In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and the biological sciences.
The terpenoids, also known as isoprenoids, are a large and diverse class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene, and the isoprene polymers called terpenes. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. Terpenoids are the largest class of plant secondary metabolites, representing about 60% of known natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists.

Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), etc. A well known monoterpene is alpha-pinene, a major component of turpentine.

Oleic acid is a fatty acid that occurs naturally in various animal and vegetable fats and oils. It is an odorless, colorless oil, although commercial samples may be yellowish. In chemical terms, oleic acid is classified as a monounsaturated omega-9 fatty acid, abbreviated with a lipid number of 18:1 cis-9. It has the formula CH3(CH2)7CH=CH(CH2)7COOH. The name derives from the Latin word oleum, which means oil. It is the most common fatty acid in nature. The salts and esters of oleic acid are called oleates.

Persicaria odorata, with common names Vietnamese coriander, Vietnamese cilantro, hot mint and Cambodian mint, is a herb whose leaves are used in Southeast Asian cooking.

Chrysopogon zizanioides, commonly known as vetiver and khus, is a perennial bunchgrass of the family Poaceae.

Caryophyllene, more formally (−)-β-caryophyllene, is a natural bicyclic sesquiterpene that is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of Syzygium aromaticum (cloves), the essential oil of Cannabis sativa, rosemary, and hops. It is usually found as a mixture with isocaryophyllene and α-humulene, a ring-opened isomer. Caryophyllene is notable for having a cyclobutane ring, as well as a trans-double bond in a 9-membered ring, both rarities in nature.
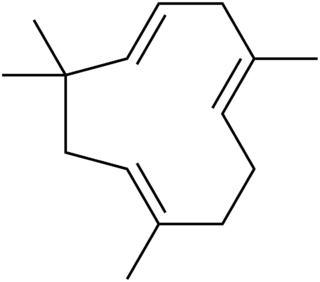
Humulene, also known as α-humulene or α-caryophyllene, is a naturally occurring monocyclic sesquiterpene (C15H24), containing an 11-membered ring and consisting of 3 isoprene units containing three nonconjugated C=C double bonds, two of them being triply substituted and one being doubly substituted. It was first found in the essential oils of Humulus lupulus (hops), from which it derives its name. Humulene is an isomer of β-caryophyllene, and the two are often found together as a mixture in many aromatic plants.

Vetivazulene is an azulene derivate obtained from vetiver oil. It is a bicyclic sesquiterpene and an isomer of guaiazulene.

The Wieland–Miescher ketone is a racemic bicyclic diketone (enedione) and is a versatile synthon which has so far been employed in the total synthesis of more than 50 natural products, predominantly sesquiterpenoids, diterpenes and steroids possessing possible biological properties including anticancer, antimicrobial, antiviral, antineurodegenerative and immunomodulatory activities. The reagent is named after two chemists from Ciba Geigy, Karl Miescher and Peter Wieland. Examples of syntheses performed using the optically active enantiomer of this diketone as a starting material are that of ancistrofuran and the Danishefsky total synthesis of Taxol.

Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be acyclic or contain rings, including many unique combinations. Biochemical modifications such as oxidation or rearrangement produce the related sesquiterpenoids.
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries.

Helenalin, or (-)-4-Hydroxy-4a,8-dimethyl-3,3a,4a,7a,8,9,9a-octahydroazuleno[6,5-b]furan-2,5-dione, is a toxic sesquiterpene lactone which can be found in several plants such as Arnica montana and Arnica chamissonis subsp. foliosa. Helenalin is responsible for the toxicity of the Arnica spp. Although toxic, helenalin possesses some in vitro anti-inflammatory and anti-neoplastic effects. Helenalin can inhibit certain enzymes, such as 5-lipoxygenase and leukotriene C4 synthase. For this reason the compound or its derivatives may have potential medical applications.

Germacrenes are a class of volatile organic hydrocarbons, specifically, sesquiterpenes. Germacrenes are typically produced in a number of plant species for their antimicrobial and insecticidal properties, though they also play a role as insect pheromones. Two prominent molecules are germacrene A and germacrene D.
The Kauffmann olefination is a chemical reaction to convert aldehydes and ketones to olefins with a terminal methylene group. This reaction was discovered by the German chemist Thomas Kauffmann and is related to the better known Tebbe olefination or Wittig reaction.
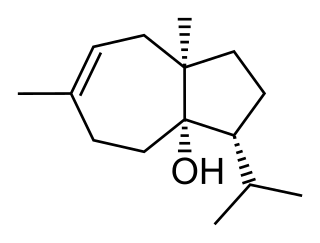
Carotol was first isolated by scientists Asahina and Tsukamoto in 1925. It is one of the primary components found in carrot seed oil comprising approximately 40% of this essential oil. This sesquiterpene alcohol is thought to be formed in carrot seeds during the vegetation period. Additionally, studies have shown that carotol may be involved in allelopathic interactions expressing activity as an antifungal, herbicidal and insecticidal agent.

Juvabione, historically known as the paper factor, is the methyl ester of todomatuic acid, both of which are sesquiterpenes (C15) found in the wood of true firs of the genus Abies. They occur naturally as part of a mixture of sesquiterpenes based upon the bisabolane scaffold. Sesquiterpenes of this family are known as insect juvenile hormone analogues (IJHA) because of their ability to mimic juvenile activity in order to stifle insect reproduction and growth. These compounds play important roles in conifers as the second line of defense against insect induced trauma and fungal pathogens.

α-Vetivone is an organic compound that is classified as a sesquiterpene. It is a major component of the oil of vetiver, which is used to prepare certain high value perfumes.
Sasanka Chandra Bhattacharyya (1918–2013) was an Indian natural product chemist and the director of Bose Institute, Kolkata. He was known for his studies on structures and configurations of terpenoids and synthesis of Vetiver Oil and natural musk. He was the vice-president of the Indian National Science Academy and was an elected fellow of the academy as well as the Indian Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1962, for his contributions to chemical sciences.














