Related Research Articles

In molecular biology, CD4 is a glycoprotein that serves as a co-receptor for the T-cell receptor (TCR). CD4 is found on the surface of immune cells such as T helper cells, monocytes, macrophages, and dendritic cells. It was discovered in the late 1970s and was originally known as leu-3 and T4 before being named CD4 in 1984. In humans, the CD4 protein is encoded by the CD4 gene.

The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.
CD8 is a transmembrane glycoprotein that serves as a co-receptor for the T-cell receptor (TCR). Along with the TCR, the CD8 co-receptor plays a role in T cell signaling and aiding with cytotoxic T cell-antigen interactions.
Biological crosstalk refers to instances in which one or more components of one signal transduction pathway affects another. This can be achieved through a number of ways with the most common form being crosstalk between proteins of signaling cascades. In these signal transduction pathways, there are often shared components that can interact with either pathway. A more complex instance of crosstalk can be observed with transmembrane crosstalk between the extracellular matrix (ECM) and the cytoskeleton.

In immunology, a Fc receptor is a protein found on the surface of certain cells – including, among others, B lymphocytes, follicular dendritic cells, natural killer cells, macrophages, neutrophils, eosinophils, basophils, human platelets, and mast cells – that contribute to the protective functions of the immune system. Its name is derived from its binding specificity for a part of an antibody known as the Fc region. Fc receptors bind to antibodies that are attached to infected cells or invading pathogens. Their activity stimulates phagocytic or cytotoxic cells to destroy microbes, or infected cells by antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity. Some viruses such as flaviviruses use Fc receptors to help them infect cells, by a mechanism known as antibody-dependent enhancement of infection.
Lck is a 56 kDa protein that is found inside specialized cells of the immune system called lymphocytes. The Lck is a member of Src kinase family (SFK), it is important for the activation of the T-cell receptor signaling in both naive T cells and effector T cells. The role of the Lck is less prominent in the activation or in the maintenance of memory CD8 T cells in comparison to CD4 T cells. In addition, the role of the lck varies among the memory T cells subsets. It seems that in mice, in the effector memory T cells (TEM) population, more than 50% of lck is present in a constitutively active conformation, whereas, only less than 20% of lck is present as active form of lck. These differences are due to differential regulation by SH2 domain–containing phosphatase-1 (Shp-1) and C-terminal Src kinase.

The B cell receptor (BCR) is a transmembrane protein on the surface of a B cell. A B cell receptor is composed of a membrane-bound immunoglobulin molecule and a signal transduction moiety. The former forms a type 1 transmembrane receptor protein, and is typically located on the outer surface of these lymphocyte cells. Through biochemical signaling and by physically acquiring antigens from the immune synapses, the BCR controls the activation of the B cell. B cells are able to gather and grab antigens by engaging biochemical modules for receptor clustering, cell spreading, generation of pulling forces, and receptor transport, which eventually culminates in endocytosis and antigen presentation. B cells' mechanical activity adheres to a pattern of negative and positive feedbacks that regulate the quantity of removed antigen by manipulating the dynamic of BCR–antigen bonds directly. Particularly, grouping and spreading increase the relation of antigen with BCR, thereby proving sensitivity and amplification. On the other hand, pulling forces delinks the antigen from the BCR, thus testing the quality of antigen binding.

Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode receptor tyrosine kinase proteins. Receptor tyrosine kinases have been shown not only to be key regulators of normal cellular processes but also to have a critical role in the development and progression of many types of cancer. Mutations in receptor tyrosine kinases lead to activation of a series of signalling cascades which have numerous effects on protein expression. Receptor tyrosine kinases are part of the larger family of protein tyrosine kinases, encompassing the receptor tyrosine kinase proteins which contain a transmembrane domain, as well as the non-receptor tyrosine kinases which do not possess transmembrane domains.
MHC-restricted antigen recognition, or MHC restriction, refers to the fact that a T cell can interact with a self-major histocompatibility complex molecule and a foreign peptide bound to it, but will only respond to the antigen when it is bound to a particular MHC molecule.

ZAP-70 is a protein normally expressed near the surface membrane of lymphocytes. It is most prominently known to be recruited upon antigen binding to the T cell receptor (TCR), and it plays a critical role in T cell signaling.

CD22, or cluster of differentiation-22, is a molecule belonging to the SIGLEC family of lectins. It is found on the surface of mature B cells and to a lesser extent on some immature B cells. Generally speaking, CD22 is a regulatory molecule that prevents the overactivation of the immune system and the development of autoimmune diseases.
An immunoreceptor tyrosine-based inhibitory motif (ITIM), is a conserved sequence of amino acids that is found intracellularly in the cytoplasmic domains of many inhibitory receptors of the non-catalytic tyrosine-phosphorylated receptor family found on immune cells. These immune cells include T cells, B cells, NK cells, dendritic cells, macrophages and mast cells. ITIMs have similar structures of S/I/V/LxYxxI/V/L, where x is any amino acid, Y is a tyrosine residue that can be phosphorylated, S is the amino acide Serine, I is the amino acid Isoleucine, and V is the amino acid Valine. ITIMs recruit SH2 domain-containing phosphatases, which inhibit cellular activation. ITIM-containing receptors often serve to target Immunoreceptor tyrosine-based activation motif(ITAM)-containing receptors, resulting in an innate inhibition mechanism within cells. ITIM bearing receptors have important role in regulation of immune system allowing negative regulation at different levels of the immune response.

CD5 is a cluster of differentiation expressed on the surface of T cells and in a subset of murine B cells known as B-1a. The expression of this receptor in human B cells has been a controversial topic and to date there is no consensus regarding the role of this receptor as a marker of human B cells. B-1 cells have limited diversity of their B-cell receptor due to their lack of the enzyme terminal deoxynucleotidyl transferase (TdT) and are potentially self-reactive. CD5 serves to mitigate activating signals from the BCR so that the B-1 cells can only be activated by very strong stimuli and not by normal tissue proteins. CD5 was used as a T-cell marker until monoclonal antibodies against CD3 were developed.

The Linker for activation of T cells, also known as linker of activated T cells or LAT, is a protein involved in the T-cell antigen receptor signal transduction pathway which in humans is encoded by the LAT gene. Alternative splicing results in multiple transcript variants encoding different isoforms.

Protein tyrosine phosphatase non-receptor type 22 (PTPN22) is a cytoplasmatic protein encoded by gene PTPN22 and a member of PEST family of protein tyrosine phosphatases. This protein is also called "PEST-domain Enriched Phosphatase" ("PEP") or "Lymphoid phosphatase" ("LYP"). The name LYP is used strictly for the human protein encoded by PTPN22, but the name PEP is used only for its mouse homolog. However, both proteins have similar biological functions and show 70% identity in amino acid sequence. PTPN22 functions as a negative regulator of T cell receptor (TCR) signaling, which maintains homeostasis of T cell compartment.

Signal regulatory protein α (SIRPα) is a regulatory membrane glycoprotein from SIRP family expressed mainly by myeloid cells and also by stem cells or neurons.
A non-receptor tyrosine kinase (nRTK) is a cytosolic enzyme that is responsible for catalysing the transfer of a phosphate group from a nucleoside triphosphate donor, such as ATP, to tyrosine residues in proteins. Non-receptor tyrosine kinases are a subgroup of protein family tyrosine kinases, enzymes that can transfer the phosphate group from ATP to a tyrosine residue of a protein (phosphorylation). These enzymes regulate many cellular functions by switching on or switching off other enzymes in a cell.

Tyrosine phosphorylation is the addition of a phosphate (PO43−) group to the amino acid tyrosine on a protein. It is one of the main types of protein phosphorylation. This transfer is made possible through enzymes called tyrosine kinases. Tyrosine phosphorylation is a key step in signal transduction and the regulation of enzymatic activity.
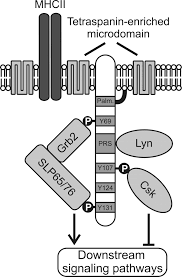
SLP65/SLP76, Csk-interacting membrane protein, termed SCIMP, belongs to family of transmembrane adaptor proteins (TRAP) which do not directly associate with a receptor, such as LAT, NTAL, LIME or LAX. SCIMP is expressed in antigen-presenting cells (APC), namely B cells, bone marrow-derived dendritic cells and macrophages.
Non-catalytic tyrosine-phosphorylated receptors (NTRs), also called immunoreceptors or Src-family kinase-dependent receptors, are a group of cell surface receptors expressed by leukocytes that are important for cell migration and the recognition of abnormal cells or structures and the initiation of an immune response. These transmembrane receptors are not grouped into the NTR family based on sequence homology, but because they share a conserved signalling pathway utilizing the same signalling motifs. A signaling cascade is initiated when the receptors bind their respective ligand resulting in cell activation. For that tyrosine residues in the cytoplasmic tail of the receptors have to be phosphorylated, hence the receptors are referred to as tyrosine-phosphorylated receptors. They are called non-catalytic receptors, as the receptors have no intrinsic tyrosine kinase activity and cannot phosphorylate their own tyrosine residues. Phosphorylation is mediated by additionally recruited kinases. A prominent member of this receptor family is the T-cell receptor.
References
- ↑ Murphy, Kenneth (2017). Janeway's immunobiology (9th ed.). New York. p. 268. ISBN 978-0815345510.
- ↑ van der Merwe, PA; Dushek, O (January 2011). "Mechanisms for T cell receptor triggering". Nature Reviews. Immunology. 11 (1): 47–55. doi:10.1038/nri2887. PMID 21127503. S2CID 22423010.
- 1 2 3 4 Davis, SJ; van der Merwe, PA (August 2006). "The kinetic-segregation model: TCR triggering and beyond". Nature Immunology. 7 (8): 803–9. doi:10.1038/ni1369. PMID 16855606. S2CID 11631728.
- ↑ Varma, R; Campi, G; Yokosuka, T; Saito, T; Dustin, ML (July 2006). "T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster". Immunity. 25 (1): 117–27. doi:10.1016/j.immuni.2006.04.010. PMC 1626533 . PMID 16860761.
- ↑ Lin, J; Weiss, A (18 August 2003). "The tyrosine phosphatase CD148 is excluded from the immunologic synapse and down-regulates prolonged T cell signaling". The Journal of Cell Biology. 162 (4): 673–82. doi: 10.1083/jcb.200303040 . PMC 2173795 . PMID 12913111.
- ↑ Irles, C; Symons, A; Michel, F; Bakker, TR; van der Merwe, PA; Acuto, O (February 2003). "CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling". Nature Immunology. 4 (2): 189–97. doi:10.1038/ni877. PMID 12496963. S2CID 31201077.
- ↑ Choudhuri, K; Parker, M; Milicic, A; Cole, DK; Shaw, MK; Sewell, AK; Stewart-Jones, G; Dong, T; Gould, KG; van der Merwe, PA (18 September 2009). "Peptide-major histocompatibility complex dimensions control proximal kinase-phosphatase balance during T cell activation". The Journal of Biological Chemistry. 284 (38): 26096–105. doi: 10.1074/jbc.M109.039966 . PMC 2758009 . PMID 19628870.
- ↑ Choudhuri, K; Wiseman, D; Brown, MH; Gould, K; van der Merwe, PA (28 July 2005). "T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand". Nature. 436 (7050): 578–82. Bibcode:2005Natur.436..578C. doi:10.1038/nature03843. PMID 16049493. S2CID 4319128.
- 1 2 Bluemel, C; Hausmann, S; Fluhr, P; Sriskandarajah, M; Stallcup, WB; Baeuerle, PA; Kufer, P (August 2010). "Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen". Cancer Immunology, Immunotherapy. 59 (8): 1197–209. doi:10.1007/s00262-010-0844-y. PMID 20309546. S2CID 10279696.
- ↑ James, SE; Greenberg, PD; Jensen, MC; Lin, Y; Wang, J; Till, BG; Raubitschek, AA; Forman, SJ; Press, OW (15 May 2008). "Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane". Journal of Immunology. 180 (10): 7028–38. doi: 10.4049/jimmunol.180.10.7028 . PMC 2585549 . PMID 18453625.
- ↑ Geppert, TD; Lipsky, PE (15 March 1987). "Accessory cell independent proliferation of human T4 cells stimulated by immobilized monoclonal antibodies to CD3". Journal of Immunology. 138 (6): 1660–6. doi:10.4049/jimmunol.138.6.1660. PMID 3102594. S2CID 26874072.
- ↑ Ma, Z; Sharp, KA; Janmey, PA; Finkel, TH (February 2008). "Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity". PLOS Biology. 6 (2): e43. doi: 10.1371/journal.pbio.0060043 . PMC 2253636 . PMID 18303949.