
A carbon sink is anything, natural or otherwise, that accumulates and stores some carbon-containing chemical compound for an indefinite period and thereby removes carbon dioxide from the atmosphere.

Plankton are the diverse collection of organisms found in water that are unable to propel themselves against a current. The individual organisms constituting plankton are called plankters. In the ocean, they provide a crucial source of food to many small and large aquatic organisms, such as bivalves, fish and whales.

The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. Carbon is the main component of biological compounds as well as a major component of many minerals such as limestone. Along with the nitrogen cycle and the water cycle, the carbon cycle comprises a sequence of events that are key to make Earth capable of sustaining life. It describes the movement of carbon as it is recycled and reused throughout the biosphere, as well as long-term processes of carbon sequestration to and release from carbon sinks. Carbon sinks in the land and the ocean each currently take up about one-quarter of anthropogenic carbon emissions each year.
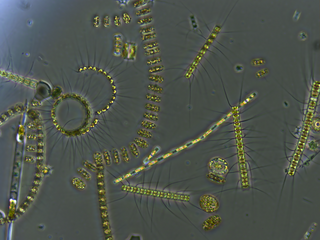
Phytoplankton are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater ecosystems. The name comes from the Greek words φυτόν, meaning 'plant', and πλαγκτός, meaning 'wanderer' or 'drifter'.

Zooplankton are heterotrophic plankton. Plankton are organisms drifting in oceans, seas, and bodies of fresh water. The word zooplankton is derived from the Greek zoon (ζῴον), meaning "animal", and planktos (πλαγκτός), meaning "wanderer" or "drifter". Individual zooplankton are usually microscopic, but some are larger and visible to the naked eye.

The biological pump, also known as the marine carbon pump, is, in its simplest form, the ocean's biologically driven sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sediments. It is the part of the oceanic carbon cycle responsible for the cycling of organic matter formed mainly by phytoplankton during photosynthesis (soft-tissue pump), as well as the cycling of calcium carbonate (CaCO3) formed into shells by certain organisms such as plankton and mollusks (carbonate pump).
High-nutrient, low-chlorophyll (HNLC) regions are regions of the ocean where the abundance of phytoplankton is low and fairly constant despite the availability of macronutrients. Phytoplankton rely on a suite of nutrients for cellular function. Macronutrients are generally available in higher quantities in surface ocean waters, and are the typical components of common garden fertilizers. Micronutrients are generally available in lower quantities and include trace metals. Macronutrients are typically available in millimolar concentrations, while micronutrients are generally available in micro- to nanomolar concentrations. In general, nitrogen tends to be a limiting ocean nutrient, but in HNLC regions it is never significantly depleted. Instead, these regions tend to be limited by low concentrations of metabolizable iron. Iron is a critical phytoplankton micronutrient necessary for enzyme catalysis and electron transport.

Iron fertilization is the intentional introduction of iron to iron-poor areas of the ocean surface to stimulate phytoplankton production. This is intended to enhance biological productivity and/or accelerate carbon dioxide sequestration from the atmosphere. Iron is a trace element necessary for photosynthesis in plants. It is highly insoluble in sea water and in a variety of locations is the limiting nutrient for phytoplankton growth. Large algal blooms can be created by supplying iron to iron-deficient ocean waters. These blooms can nourish other organisms.

Carbon sequestration is the process of storing carbon in a carbon pool. Carbon dioxide is naturally captured from the atmosphere through biological, chemical, and physical processes. These changes can be accelerated through changes in land use and agricultural practices, such as converting crop land into land for non-crop fast growing plants. Artificial processes have been devised to produce similar effects, including large-scale, artificial capture and sequestration of industrially produced CO
2 using subsurface saline aquifers, reservoirs, ocean water, aging oil fields, or other carbon sinks, bio-energy with carbon capture and storage, biochar, enhanced weathering, and direct air capture when combined with storage.

Ocean fertilization or ocean nourishment is a type of climate engineering based on the purposeful introduction of plant nutrients to the upper ocean to increase marine food production and to remove carbon dioxide from the atmosphere. A number of techniques, including fertilization by the micronutrient iron or with nitrogen and phosphorus, have been proposed. But research in the early 2020s suggested that it could only permanently sequester a small amount of carbon. Therefore, there is no major future in its role to sequester carbon.

Carbon dioxide removal (CDR), also known as negative CO2 emissions, is a process in which carbon dioxide gas is removed from the atmosphere and sequestered for long periods of time. Similarly, greenhouse gas removal (GGR) or negative greenhouse gas emissions is the removal of greenhouse gases (GHGs) from the atmosphere by deliberate human activities, i.e., in addition to the removal that would occur via natural carbon cycle or atmospheric chemistry processes. In the context of net zero greenhouse gas emissions targets, CDR is increasingly integrated into climate policy, as a new element of mitigation strategies. CDR and GGR methods are also known as negative emissions technologies (NET), and may be cheaper than preventing some agricultural greenhouse gas emissions.

In the deep ocean, marine snow is a continuous shower of mostly organic detritus falling from the upper layers of the water column. It is a significant means of exporting energy from the light-rich photic zone to the aphotic zone below, which is referred to as the biological pump. Export production is the amount of organic matter produced in the ocean by primary production that is not recycled (remineralised) before it sinks into the aphotic zone. Because of the role of export production in the ocean's biological pump, it is typically measured in units of carbon. The term was first coined by the explorer William Beebe as he observed it from his bathysphere. As the origin of marine snow lies in activities within the productive photic zone, the prevalence of marine snow changes with seasonal fluctuations in photosynthetic activity and ocean currents. Marine snow can be an important food source for organisms living in the aphotic zone, particularly for organisms which live very deep in the water column.

Bacterioplankton refers to the bacterial component of the plankton that drifts in the water column. The name comes from the Ancient Greek word πλανκτος, meaning "wanderer" or "drifter", and bacterium, a Latin term coined in the 19th century by Christian Gottfried Ehrenberg. They are found in both seawater and freshwater.

The North Pacific Subtropical Gyre (NPSG) is the largest contiguous ecosystem on earth. In oceanography, a subtropical gyre is a ring-like system of ocean currents rotating clockwise in the Northern Hemisphere and counterclockwise in the Southern Hemisphere caused by the Coriolis Effect. They generally form in large open ocean areas that lie between land masses.

Blue carbon is carbon sequestration by the world's oceanic and coastal ecosystems, mostly by algae, seagrasses, macroalgae, mangroves, salt marshes and other plants in coastal wetlands. This occurs through plant growth and the accumulation and burial of organic matter in the soil. Because oceans cover 70% of the planet, ocean ecosystem restoration has the greatest blue carbon development potential. Research is ongoing, but in some cases it has been found that these types of ecosystems remove far more carbon than terrestrial forests, and store it for millennia.
Syed Wajih Ahmad Naqvi is an Indian marine scientist and the former director of the National Institute of Oceanography. His work has concentrated in oceanic water chemistry, biogeochemistry, and chemical interrelations with living organisms. He has also performed research on freshwater ecosystems. He was the chief Indian scientist of LOHAFEX, an ocean iron fertilization experiment jointly planned by the Council of Scientific Industrial Research (CSIR), India, and Helmholtz Foundation, Germany.

Particulate organic matter (POM) is a fraction of total organic matter operationally defined as that which does not pass through a filter pore size that typically ranges in size from 0.053 to 2 millimeters.

Bettina Meyer is a German Antarctic researcher, best known for her work on the ecology and physiology of invertebrates in the pelagic zone. She is the head of the ecophysiology of pelagic key species working group at the Alfred Wegener Institute for Polar and Marine Research (AWI).

Carbon farming is a name for a variety of agricultural methods aimed at sequestering atmospheric carbon into the soil and in crop roots, wood and leaves. The aim of carbon farming is to increase the rate at which carbon is sequestered into soil and plant material with the goal of creating a net loss of carbon from the atmosphere. Increasing a soil's organic matter content can aid plant growth, increase total carbon content, improve soil water retention capacity and reduce fertilizer use. As of 2016, variants of carbon farming reached hundreds of millions of hectares globally, of the nearly 5 billion hectares (1.2×1010 acres) of world farmland. In addition to agricultural activities, forests management is also a tool that is used in carbon farming. The practice of carbon farming is often done by individual land owners who are given incentive to use and to integrate methods that will sequester carbon through policies created by governments. Carbon farming methods will typically have a cost, meaning farmers and land-owners need a way to profit from the use of carbon farming, and different governments will have different programs.

Ocean storage of carbon dioxide (CO2) is a method of carbon sequestration. The concept of storing carbon dioxide in the ocean was first proposed by Italian physicist Cesare Marchetti in his 1976 paper "On Geoengineering and the carbon dioxide problem." Since then, the concept of sequestering atmospheric carbon dioxide in the world's oceans has been investigated by scientists, engineers, and environmental activists. 39,000 GtC (gigatonnes of carbon) currently reside in the oceans while only 750 GtC are in the atmosphere.
















