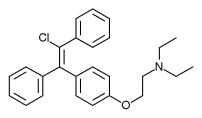
Enclomifene (INNTooltip International Nonproprietary Name), or enclomiphene (USANTooltip United States Adopted Name), a nonsteroidal selective estrogen receptor modulator of the triphenylethylene group acts by antagonizing the estrogen receptor (ER) in the pituitary gland, which reduces negative feedback by estrogen on the hypothalamic-pituitary-gonadal axis, thereby increasing gonadotropin secretion and hence gonadal production of testosterone. It is one of the two stereoisomers of clomifene, which itself is a mixture of 38% zuclomifene and 62% enclomifene. Enclomifene is the (E)-stereoisomer of clomifene, while zuclomifene is the (Z)-stereoisomer. Whereas zuclomifene is more estrogenic, enclomifene is more antiestrogenic. In accordance, unlike enclomifene, zuclomifene is antigonadotropic due to activation of the ER and reduces testosterone levels in men. As such, isomerically pure enclomifene is more favorable than clomifene as a progonadotropin for the treatment of male hypogonadism.

Eltoprazine is a serotonergic drug of the phenylpiperazine class which is described as a serenic or antiaggressive agent. It acts as an agonist of the serotonin 5-HT1A and 5-HT1B receptors and as an antagonist of the serotonin 5-HT2C receptor. The drug is closely related to fluprazine and batoprazine, which are similarly acting agents, and is also a known chemical precursor to S-15535 and lecozotan. Eltoprazine is or was under development for the treatment of aggression, attention deficit hyperactivity disorder (ADHD), cognition disorders, and drug-induced dyskinesia, but no recent development has been reported for these indications as of February 2022. It was also under development for the treatment of psychotic disorders, but development for this indication was discontinued. Eltoprazine was originated by Solvay and was developed by Elto Pharma, PsychoGenics, and Solvay.

Acceleron Pharma, Inc. is an American clinical stage biopharmaceutical company based in Cambridge, Massachusetts with a broad focus on developing medicines that regulate the transforming growth factor beta (TGF-β) superfamily of proteins, which play fundamental roles in the growth and repair of cells and tissues such as red blood cells, muscle, bone, and blood vessels.
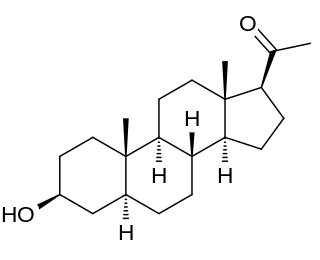
Isopregnanolone, also known as isoallopregnanolone and epiallopregnanolone, as well as sepranolone, and as 3β-hydroxy-5α-pregnan-20-one or 3β,5α-tetrahydroprogesterone (3β,5α-THP), is an endogenous neurosteroid and a natural 3β-epimer of allopregnanolone. It has been reported to act as a subunit-selective negative allosteric modulator of the GABAA receptor, and antagonizes in animals and humans some but not all of the GABAA receptor-mediated effects of allopregnanolone, such as anesthesia, sedation, and reduced saccadic eye movements, but not learning impairment. Isopregnanolone has no hormonal effects and appears to have no effect on the GABAA receptor by itself; it selectively antagonizes allopregnanolone and does not affect the effects of other types of GABAA receptor positive allosteric modulators such as benzodiazepines or barbiturates.

Funapide (INN) is a novel analgesic under development by Xenon Pharmaceuticals for the treatment of a variety of chronic pain conditions, including osteoarthritis, neuropathic pain, postherpetic neuralgia, and erythromelalgia, as well as dental pain. It acts as a small-molecule Nav1.7 and Nav1.8 voltage-gated sodium channel blocker. Funapide is being evaluated in humans in both oral and topical formulations, and as of July 2014, has reached phase IIb clinical trials.

DSP-2230 is a selective small-molecule Nav1.7 and Nav1.8 voltage-gated sodium channel blocker which is under development by Dainippon Sumitomo Pharma for the treatment of neuropathic pain. As of June 2014, it is in phase I/phase II clinical trials.

Toludesvenlafaxine, also formerly known as ansofaxine and sold under the brand name Ruoxinlin, is an antidepressant which is approved for the treatment of major depressive disorder in China. It is also under development for use in other countries like the United States. It is a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) and was developed by Luye Pharma Group.

Ralaniten acetate is a first-in-class antiandrogen that targets the N-terminal domain (NTD) of the androgen receptor (AR) developed by ESSA Pharmaceuticals and was under investigation for the treatment of prostate cancer. This mechanism of action is believed to allow the drug to block signaling from the AR and its splice variants. EPI-506 is a derivative of bisphenol A and a prodrug of ralaniten (EPI-002), one of the four stereoisomers of EPI-001, and was developed as a successor of EPI-001. The drug reached phase I/II prior to the discontinuation of its development. It showed signs of efficacy in the form of prostatic specific antigen (PSA) decreases (4–29%) predominantly at higher doses (≥1,280 mg) in some patients but also caused side effects and was discontinued by its developer in favor of next-generation AR NTD inhibitors with improved potency and tolerability.

Fispemifene is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed for the treatment of male hypogonadism but was abandoned and never marketed. It reached phase II clinical trials for this indication before development was terminated in March 2016. The drug failed to achieve statistical significance on key effectiveness endpoints in clinical trials and was discontinued by its developer for strategic reasons.

Fezolinetant, sold under the brand name Veozah, is a medication used for the treatment of hot flashes (vasomotor symptoms) due to menopause. It is a small-molecule, orally active, selective neurokinin-3 (NK3) receptor antagonist which is under development by for the treatment of sex hormone-related disorders. It is developed by Astellas Pharma which acquired it from Ogeda (formerly Euroscreen) in April 2017.

Apararenone (INN) is a nonsteroidal antimineralocorticoid which is under development by Mitsubishi Tanabe Pharma for the treatment of diabetic nephropathies and non-alcoholic steatohepatitis. It was also previously being developed for the treatment of hypertension, but development was discontinued for this indication. Apararenone acts as a highly selective antagonist of the mineralocorticoid receptor, the receptor for aldosterone. As of 2017, it is in phase II clinical trials.
Testosterone/dutasteride is a combination formulation of testosterone, an androgen, and dutasteride, a 5α-reductase inhibitor, which was under development by GlaxoSmithKline for the treatment of hypogonadism in men in the 2000s. It reached phase II clinical trials prior to the discontinuation of its development.
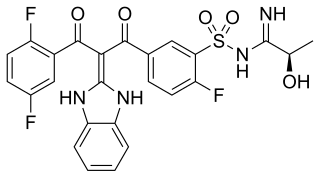
Opigolix is a small-molecule, non-peptide, orally active gonadotropin-releasing hormone antagonist which was under development by Astellas Pharma for the treatment of endometriosis and rheumatoid arthritis. It was also under investigation for the treatment of prostate cancer. It reached phase II clinical trials for both endometriosis and rheumatoid arthritis prior to the discontinuation of its development in April 2018.

Ultragenyx is an American biopharmaceutical company involved in the research and development of novel products for treatment of rare and ultra-rare genetic diseases for which there are typically no approved treatments and high unmet medical need. The company works with multiple drug modalities including biologics, small molecule, gene therapies, and ASO and mRNAs in the disease categories of bone, endocrine, metabolic, muscle and CNS diseases.

MVT-602 is a kisspeptin receptor agonist which is under development for the treatment of female infertility and hypogonadism. It has been found to increase luteinizing hormone levels in premenopausal women. As of March 2021, MVT-602 is in phase 2 clinical trials for the treatment of female infertility and hypogonadism. It was also under development for the treatment of prostate cancer, but development for this indication was discontinued.
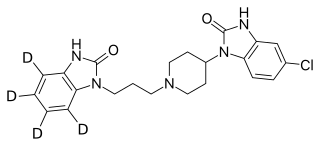
Deudomperidone (developmental code name CIN-102; also known as deuterated domperidone) is a dopamine antagonist medication which is under development in the United States for the treatment of gastroparesis. It acts as a selective dopamine D2 and D3 receptor antagonist and has peripheral selectivity. Deudomperidone is a deuterated form of domperidone, and it is suggested that deudomperidone may have improved efficacy, tolerability, and pharmacokinetics compared to domperidone. As of January 2022, deudomperidone is in phase 2 clinical trials for the treatment of gastroparesis.

Sunobinop is a nociceptin receptor partial agonist and opioid receptor pan-antagonist. As of March 2022, it is under investigation for the treatment of insomnia, fibromyalgia, and overactive bladder.

Posovolone is a synthetic neurosteroid which was under development as a sedative/hypnotic medication for the treatment of insomnia. It is orally active and acts as a GABAA receptor positive allosteric modulator. In animals, posovolone shows anticonvulsant, anxiolytic-like, ataxic, and sleep-promoting effects and appeared to produce effects similar to those of pregnanolone. Development of the agent was started by 1999 and appears to have been discontinued by 2007. In 2021, an INNTooltip International Nonproprietary Name was registered for posovolone with the descriptor of "antidepressant". Posovolone was originally developed by Purdue Pharma.

Darinaparsin is a drug for the treatment of various types of cancer. It is an arsenic-containing derivative of glutathione.


















