Related Research Articles

In molecular biology, a transcription factor (TF) is a protein that controls the rate of transcription of genetic information from DNA to messenger RNA, by binding to a specific DNA sequence. The function of TFs is to regulate—turn on and off—genes in order to make sure that they are expressed in the desired cells at the right time and in the right amount throughout the life of the cell and the organism. Groups of TFs function in a coordinated fashion to direct cell division, cell growth, and cell death throughout life; cell migration and organization during embryonic development; and intermittently in response to signals from outside the cell, such as a hormone. There are approximately 1600 TFs in the human genome. Transcription factors are members of the proteome as well as regulome.

In biology, epigenetics is the study of heritable traits, or a stable change of cell function, that happen without changes to the DNA sequence. The Greek prefix epi- in epigenetics implies features that are "on top of" or "in addition to" the traditional genetic mechanism of inheritance. Epigenetics usually involves a change that is not erased by cell division, and affects the regulation of gene expression. Such effects on cellular and physiological phenotypic traits may result from environmental factors, or be part of normal development. Epigenetic factors can also lead to cancer.

Gene expression is the process by which information from a gene is used in the synthesis of a functional gene product that enables it to produce end products, proteins or non-coding RNA, and ultimately affect a phenotype. These products are often proteins, but in non-protein-coding genes such as transfer RNA (tRNA) and small nuclear RNA (snRNA), the product is a functional non-coding RNA. The process of gene expression is used by all known life—eukaryotes, prokaryotes, and utilized by viruses—to generate the macromolecular machinery for life.
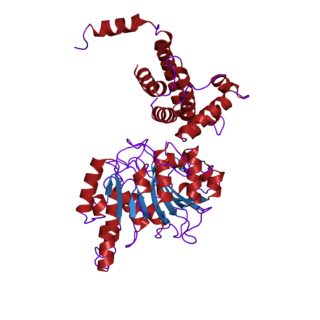
In biochemistry, the DNA methyltransferase family of enzymes catalyze the transfer of a methyl group to DNA. DNA methylation serves a wide variety of biological functions. All the known DNA methyltransferases use S-adenosyl methionine (SAM) as the methyl donor.

Regulation of gene expression, or gene regulation, includes a wide range of mechanisms that are used by cells to increase or decrease the production of specific gene products. Sophisticated programs of gene expression are widely observed in biology, for example to trigger developmental pathways, respond to environmental stimuli, or adapt to new food sources. Virtually any step of gene expression can be modulated, from transcriptional initiation, to RNA processing, and to the post-translational modification of a protein. Often, one gene regulator controls another, and so on, in a gene regulatory network.

EGR-1 or NGFI-A is a protein that in humans is encoded by the EGR1 gene.
Deoxyribozymes, also called DNA enzymes, DNAzymes, or catalytic DNA, are DNA oligonucleotides that are capable of performing a specific chemical reaction, often but not always catalytic. This is similar to the action of other biological enzymes, such as proteins or ribozymes . However, in contrast to the abundance of protein enzymes in biological systems and the discovery of biological ribozymes in the 1980s, there is only little evidence for naturally occurring deoxyribozymes. Deoxyribozymes should not be confused with DNA aptamers which are oligonucleotides that selectively bind a target ligand, but do not catalyze a subsequent chemical reaction.

Transcription factor Sp1, also known as specificity protein 1* is a protein that in humans is encoded by the SP1 gene.
Dz13 is an experimental treatment developed by scientists at the University of New South Wales. The drug aims to combat a range of illnesses, including skin cancer, restenosis, arthritis and macular degeneration. Trials of Dz13 were suspended in 2013.

Nuclear factor erythroid 2-related factor 2 (NRF2), also known as nuclear factor erythroid-derived 2-like 2, is a transcription factor that in humans is encoded by the NFE2L2 gene. NRF2 is a basic leucine zipper (bZIP) protein that may regulate the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation, according to preliminary research. In vitro, NRF2 binds to antioxidant response elements (AREs) in the promoter regions of genes encoding cytoprotective proteins. NRF2 induces the expression of heme oxygenase 1 in vitro leading to an increase in phase II enzymes. NRF2 also inhibits the NLRP3 inflammasome.

Forkhead box O3, also known as FOXO3 or FOXO3a, is a human protein encoded by the FOXO3 gene.

Enhancer of zeste homolog 2 (EZH2) is a histone-lysine N-methyltransferase enzyme encoded by EZH2 gene, that participates in histone methylation and, ultimately, transcriptional repression. EZH2 catalyzes the addition of methyl groups to histone H3 at lysine 27, by using the cofactor S-adenosyl-L-methionine. Methylation activity of EZH2 facilitates heterochromatin formation thereby silences gene function. Remodeling of chromosomal heterochromatin by EZH2 is also required during cell mitosis.

DNA topoisomerase 2-beta is an enzyme that in humans is encoded by the TOP2B gene.

Activating transcription factor 4 , also known as ATF4, is a protein that in humans is encoded by the ATF4 gene.

Histone deacetylase 9 is an enzyme that in humans is encoded by the HDAC9 gene.

Vpr is a Human immunodeficiency virus gene and protein product. Vpr stands for "Viral Protein R". Vpr, a 96 amino acid 14-kDa protein, plays an important role in regulating nuclear import of the HIV-1 pre-integration complex, and is required for virus replication and enhanced gene expression from provirus in dividing or non-dividing cells such as T cells or macrophages. Vpr also induces G2 cell cycle arrest and apoptosis in proliferating cells, which can result in immune dysfunction.
Merlin Crossley, is an Australian molecular biologist, university teacher, and administrator. He is Deputy Vice-Chancellor (DVC) Academic Quality at the University of New South Wales.

Epigenome editing or epigenome engineering is a type of genetic engineering in which the epigenome is modified at specific sites using engineered molecules targeted to those sites. Whereas gene editing involves changing the actual DNA sequence itself, epigenetic editing involves modifying and presenting DNA sequences to proteins and other DNA binding factors that influence DNA function. By "editing” epigenomic features in this manner, researchers can determine the exact biological role of an epigenetic modification at the site in question.
Michelle Haber is an Australian cancer researcher in the field of childhood cancer research.
H3K9me2 is an epigenetic modification to the DNA packaging protein Histone H3. It is a mark that indicates the di-methylation at the 9th lysine residue of the histone H3 protein. H3K9me2 is strongly associated with transcriptional repression. H3K9me2 levels are higher at silent compared to active genes in a 10kb region surrounding the transcriptional start site. H3K9me2 represses gene expression both passively, by prohibiting acetylation as therefore binding of RNA polymerase or its regulatory factors, and actively, by recruiting transcriptional repressors. H3K9me2 has also been found in megabase blocks, termed Large Organised Chromatin K9 domains (LOCKS), which are primarily located within gene-sparse regions but also encompass genic and intergenic intervals. Its synthesis is catalyzed by G9a, G9a-like protein, and PRDM2. H3K9me2 can be removed by a wide range of histone lysine demethylases (KDMs) including KDM1, KDM3, KDM4 and KDM7 family members. H3K9me2 is important for various biological processes including cell lineage commitment, the reprogramming of somatic cells to induced pluripotent stem cells, regulation of the inflammatory response, and addiction to drug use.
References
- 1 2 3 4 "To crack the maze". Sydney Morning Herald. 27 April 2006.
- 1 2 3 4 5 "Levon M. Khachigian: IJO Editorial Academy (September 2012)".
- 1 2 3 4 5 "LM Khachigian website".
- ↑ "Wide role for new drug targeting skin cancer gene (Sydney Morning Herald 7 May 2013)". 6 May 2013.
- ↑ "Gene result a touch of shear brilliance". Sydney Morning Herald. 14 August 2003.
- 1 2 Khachigian, Levon. "Tall Poppies in Flight" (PDF). Australian Institute of Policy and Science.
- ↑ Khachigian, LM; et al. (1996). "Egr-1-induced endothelial gene expression: a common theme in vascular injury". Science. 271 (5254): 1427–31. Bibcode:1996Sci...271.1427K. doi:10.1126/science.271.5254.1427. PMID 8596917. S2CID 36935337.
- ↑ Khachigian, LM (2006). "Early growth response-1 in cardiovascular pathobiology". Circ Res. 98 (2): 186–91. doi: 10.1161/01.res.0000200177.53882.c3 . PMID 16456111.
- ↑ Tan, NY; et al. (2009). "Sp1 phosphorylation and its regulation of gene transcription". Mol Cell Biol. 29 (10): 2483–2488. doi:10.1128/mcb.01828-08. PMC 2682032 . PMID 19273606.
- ↑ Zhang, N; et al. (2012). "Repression of PDGF-R-alpha after cellular injury involves TNF-alpha, formation of a c-Fos-YY1 complex, and negative regulation by HDAC". Am J Physiol Cell Physiol. 302 (11): C1590-8. doi:10.1152/ajpcell.00429.2011. PMID 22322974.
- ↑ Khachigian, LM (2016). "Early growth response-1 in the pathogenesis of cardiovascular disease". J Mol Med. 94 (7): 747–753. doi:10.1007/s00109-016-1428-x. PMID 27251707. S2CID 15905744.
- ↑ Forrest, AR; et al. (2014). "A promoter-level mammalian expression atlas". Nature. 507 (7493): 462–470. Bibcode:2014Natur.507..462T. doi:10.1038/nature13182. PMC 4529748 . PMID 24670764.
- ↑ Arner, E; et al. (2015). "Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells". Science. 347 (6225): 1010–1014. Bibcode:2015Sci...347.1010A. doi:10.1126/science.1259418. PMC 4681433 . PMID 25678556.
- ↑ Khachigian, LM (2000). "Catalytic DNAs as potential therapeutic agents and sequence-specific molecular tools to dissect biological function". J Clin Invest. 106 (10): 1189–95. doi:10.1172/jci11620. PMC 381443 . PMID 11086018.
- ↑ Santiago, FS; et al. (1999). "New DNA enzyme targeting Egr-1 mRNA inhibits vascular smooth muscle proliferation and regrowth after injury". Nature Medicine. 5 (11): 1264–1269. doi:10.1038/15215. PMID 10545992. S2CID 9566888.
- ↑ Cho, EA; et al. (2013). "Safety and tolerability of an intratumorally injected DNAzyme, Dz13, in patients with nodular basal-cell carcinoma: a phase 1 first-in-human trial (DISCOVER)". The Lancet. 381 (9880): 1835–1843. doi:10.1016/s0140-6736(12)62166-7. PMC 3951714 . PMID 23660123.
- ↑ Krug, N; et al. (2015). "Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme". N Engl J Med. 372 (21): 1987–95. doi:10.1056/nejmoa1411776. hdl: 1854/LU-6862585 . PMID 25981191.
- ↑ Cao, Y; et al. (2014). "Therapeutic evaluation of Epstein-Barr virus-encoded latent membrane protein-1 targeted DNAzyme for treating of nasopharyngeal carcinomas". Mol Ther. 22 (2): 371–7. doi:10.1038/mt.2013.257. PMC 3916047 . PMID 24322331.
- ↑ "Dz13 drug targets skin cancer in clinical trial (Asian Scientist 13 May 2013)". 13 May 2013.
- ↑ Khachigian, LM (2007). "Science never sleeps: Tucker Collins MD, PhD". Endothelium. 14 (4–5): 173–174. doi:10.1080/10623320701670026.
- ↑ "First class honours (UNSW 12 June 2007)". 12 June 2007.
- ↑ "F1000 Prime Drug Discovery & Design (14 June 2012)".
- ↑ "Novel and pioneering research offers hope for cardiovascular disease (2006)".
- ↑ "ABC News (18 April 2014)". Australian Broadcasting Corporation . 17 April 2014.
- ↑ "Khachigian statement (11 August 2013)" (PDF). Australian Broadcasting Corporation .
- 1 2 "UNSW statement (12 August 2013)". 12 August 2013.
- ↑ "Retraction Watch Database".
- ↑ "UNSW public statement (26 November 2015)".
- ↑ "UNSW clears Levon Khachigian of all allegations of research misconduct (22 December 2015)".
- 1 2 "UNSW skin cancer researcher Levon Khachigian". Australian Broadcasting Corporation . 16 October 2019.
- ↑ Ryan, Jackson (September 2023). "Science fiction in university labs?". The Monthly.