Related Research Articles

Alessandro Giuseppe Antonio Anastasio Volta was an Italian physicist and chemist who was a pioneer of electricity and power and is credited as the inventor of the electric battery and the discoverer of methane. He invented the voltaic pile in 1799, and reported the results of his experiments in 1800 in a two-part letter to the president of the Royal Society. With this invention Volta proved that electricity could be generated chemically and debunked the prevalent theory that electricity was generated solely by living beings. Volta's invention sparked a great amount of scientific excitement and led others to conduct similar experiments, which eventually led to the development of the field of electrochemistry.

Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwell's equations. Various common phenomena are related to electricity, including lightning, static electricity, electric heating, electric discharges and many others.
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to move a test charge between the two points. In the International System of Units (SI), the derived unit for voltage is named volt.

The voltaic pile was the first electrical battery that could continuously provide an electric current to a circuit. It was invented by Italian chemist Alessandro Volta, who published his experiments in 1799. Its invention can be traced back to an argument between Volta and Luigi Galvani, Volta’s fellow Italian scientist who had conducted experiments on frogs' legs. The voltaic pile then enabled a rapid series of other discoveries including the electrical decomposition (electrolysis) of water into oxygen and hydrogen by William Nicholson and Anthony Carlisle (1800) and the discovery or isolation of the chemical elements sodium (1807), potassium (1807), calcium (1808), boron (1808), barium (1808), strontium (1808), and magnesium (1808) by Humphry Davy.

The volt is the unit of electric potential, electric potential difference (voltage), and electromotive force in the International System of Units (SI). It is named after the Italian physicist Alessandro Volta (1745–1827).

Direct current (DC) is one-directional flow of electric charge. An electrochemical cell is a prime example of DC power. Direct current may flow through a conductor such as a wire, but can also flow through semiconductors, insulators, or even through a vacuum as in electron or ion beams. The electric current flows in a constant direction, distinguishing it from alternating current (AC). A term formerly used for this type of current was galvanic current.
Timeline of electromagnetism and classical optics lists, within the history of electromagnetism, the associated theories, technology, and events.

Galvanism is a term invented by the late 18th-century physicist and chemist Alessandro Volta to refer to the generation of electric current by chemical action. The term also came to refer to the discoveries of its namesake, Luigi Galvani, specifically the generation of electric current within biological organisms and the contraction/convulsion of biological muscle tissue upon contact with electric current. While Volta theorized and later demonstrated the phenomenon of his "Galvanism" to be replicable with otherwise inert materials, Galvani thought his discovery to be a confirmation of the existence of "animal electricity," a vital force which gave life to organic matter.

Luigi Galvani was an Italian physician, physicist, biologist and philosopher, who studied animal electricity. In 1780, he discovered that the muscles of dead frogs' legs twitched when struck by an electrical spark. This was an early study of bioelectricity, following experiments by John Walsh and Hugh Williamson.

A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous Oxidation-Reduction reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane.

Nicholas Joseph Callan was an Irish Catholic priest and physicist. He was professor of natural philosophy at Maynooth College in County Kildare from 1834, and is best known for his work on the induction coil.
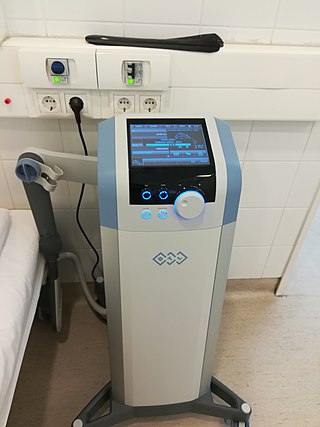
Electrotherapy is the use of electrical energy as a medical treatment. In medicine, the term electrotherapy can apply to a variety of treatments, including the use of electrical devices such as deep brain stimulators for neurological disease. The term has also been applied specifically to the use of electric current to speed wound healing. Additionally, the term "electrotherapy" or "electromagnetic therapy" has also been applied to a range of alternative medical devices and treatments.
Electrochemistry, a branch of chemistry, went through several changes during its evolution from early principles related to magnets in the early 16th and 17th centuries, to complex theories involving conductivity, electric charge and mathematical methods. The term electrochemistry was used to describe electrical phenomena in the late 19th and 20th centuries. In recent decades, electrochemistry has become an area of current research, including research in batteries and fuel cells, preventing corrosion of metals, the use of electrochemical cells to remove refractory organics and similar contaminants in wastewater electrocoagulation and improving techniques in refining chemicals with electrolysis and electrophoresis.

The history of electromagnetic theory begins with ancient measures to understand atmospheric electricity, in particular lightning. People then had little understanding of electricity, and were unable to explain the phenomena. Scientific understanding into the nature of electricity grew throughout the eighteenth and nineteenth centuries through the work of researchers such as Coulomb, Ampère, Faraday and Maxwell.

The Pulvermacher chain, or in full as it was sold the Pulvermacher hydro-electric chain, was a type of voltaic battery sold in the second half of the 19th century for medical applications. Its chief market was amongst the numerous quack practitioners who were taking advantage of the popularity of the relatively new treatment of electrotherapy, or "electrification" as it was then known. Its unique selling point was its construction of numerous linked cells, rendering it mechanically flexible. A variant intended to be worn wrapped on parts of the body for long periods was known as Pulvermacher's galvanic chain or electric belt.

A frog battery is an electrochemical battery consisting of a number of dead frogs, which form the cells of the battery connected in a series arrangement. It is a kind of biobattery. It was used in early scientific investigations of electricity and academic demonstrations.
The frog galvanoscope was a sensitive electrical instrument used to detect voltage in the late 18th and 19th centuries. It consists of a skinned frog's leg with electrical connections to a nerve. The instrument was invented by Luigi Galvani and improved by Carlo Matteucci.
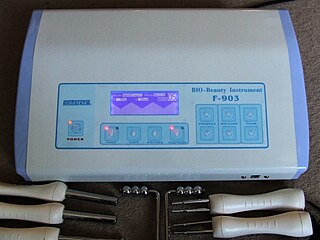
Cosmetic electrotherapy is a range of beauty treatments that uses low electric currents passed through the skin to produce several therapeutic effects such as muscle toning in the body and micro-lifting of the face. It is based on electrotherapy, which has been researched and accepted in the field of rehabilitation, though the "scientific and medical communities have tended to sideline or dismiss the use of electrotherapy for healthy muscles".

Franklin's electrostatic machine is a high-voltage static electricity-generating device used by Benjamin Franklin in the mid-18th century for research into electrical phenomena. Its key components are a glass globe which turned on an axis via a crank, a cloth pad in contact with the spinning globe, a set of metal needles to conduct away the charge developed on the globe by its friction with the pad, and a Leyden jar – a high-voltage capacitor – to accumulate the charge. Franklin's experiments with the machine eventually led to new theories about electricity and inventing the lightning rod.