
Desipramine, sold under the brand name Norpramin among others, is a tricyclic antidepressant (TCA) used in the treatment of depression. It acts as a relatively selective norepinephrine reuptake inhibitor, though it does also have other activities such as weak serotonin reuptake inhibitory, α1-blocking, antihistamine, and anticholinergic effects. The drug is not considered a first-line treatment for depression since the introduction of selective serotonin reuptake inhibitor (SSRI) antidepressants, which have fewer side effects and are safer in overdose.
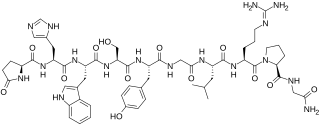
Gonadorelin is a gonadotropin-releasing hormone agonist which is used in fertility medicine and to treat amenorrhea and hypogonadism. It is also used in veterinary medicine. The medication is a form of the endogenous GnRH and is identical to it in chemical structure. It is given by injection into a blood vessel or fat or as a nasal spray.
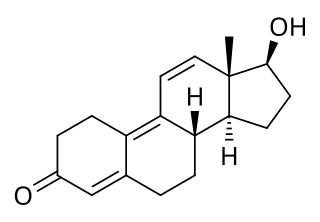
Trenbolone is an androgen and anabolic steroid (AAS) of the nandrolone group which itself was never marketed. Trenbolone ester prodrugs, including trenbolone acetate and trenbolone hexahydrobenzylcarbonate, are or have been marketed for veterinary and clinical use. Trenbolone acetate is used in veterinary medicine in livestock to increase muscle growth and appetite, while trenbolone hexahydrobenzylcarbonate was formerly used clinically in humans but is now no longer marketed. In addition, although it is not approved for clinical or veterinary use, trenbolone enanthate is sometimes sold on the black market under the nickname Trenabol.

Etoperidone, associated with several brand names, is an atypical antidepressant which was developed in the 1970s and either is no longer marketed or was never marketed. It is a phenylpiperazine related to trazodone and nefazodone in chemical structure and is a serotonin antagonist and reuptake inhibitor (SARI) similarly to them.
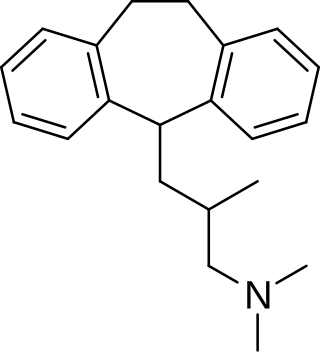
Butriptyline, sold under the brand name Evadyne among others, is a tricyclic antidepressant (TCA) that has been used in the United Kingdom and several other European countries for the treatment of depression but appears to no longer be marketed. Along with trimipramine, iprindole, and amoxapine, it has been described as an "atypical" or "second-generation" TCA due to its relatively late introduction and atypical pharmacology. It was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.

Norethandrolone, sold under the brand names Nilevar and Pronabol among others, is an androgen and anabolic steroid (AAS) medication which has been used to promote muscle growth and to treat severe burns, physical trauma, and aplastic anemia but has mostly been discontinued. It is still available for use in France however. It is taken by mouth.

Norgestrienone, sold under the brand names Ogyline, Planor, and Miniplanor, is a progestin medication which has been used in birth control pills, sometimes in combination with ethinylestradiol. It was developed by Roussel Uclaf and has been registered for use only in France. Under the brand name Planor, it has been marketed in France as 2 mg norgestrienone and 50 μg ethinylestradiol tablets. It is taken by mouth.

Mesulergine (INNTooltip International Nonproprietary Name) (developmental code name CU-32085) is a drug of the ergoline group which was never marketed. It acts on serotonin and dopamine receptors. Specifically, it is an agonist of dopamine D2-like receptors and serotonin 5-HT6 receptors and an antagonist of serotonin 5-HT2A, 5-HT2B, 5-HT2C, and 5-HT7 receptors.. It also has affinity for the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1F, and 5-HT5A receptors. The compound had entered clinical trials for the treatment of Parkinson's disease; however, further development was halted due to adverse histological abnormalities in rats. It was also investigated for the treatment of hyperprolactinemia (high prolactin levels).

Nafoxidine or nafoxidine hydrochloride is a nonsteroidal selective estrogen receptor modulator (SERM) or partial antiestrogen of the triphenylethylene group that was developed for the treatment of advanced breast cancer by Upjohn in the 1970s but was never marketed. It was developed at around the same time as tamoxifen and clomifene, which are also triphenylethylene derivatives. The drug was originally synthesized by the fertility control program at Upjohn as a postcoital contraceptive, but was subsequently repurposed for the treatment of breast cancer. Nafoxidine was assessed in clinical trials in the treatment of breast cancer and was found to be effective. However, it produced side effects including ichthyosis, partial hair loss, and phototoxicity of the skin in almost all patients, and this resulted in the discontinuation of its development.
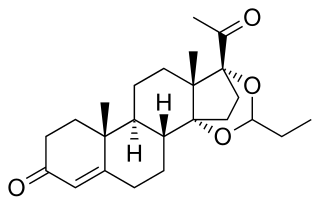
Proligestone, sold under the brand names Covinan and Delvosteron, is a progestin medication which is used in veterinary medicine.

Norvinisterone, sold under the brand names Neoprogestin and Nor-Progestelea, is a progestin and androgen/anabolic steroid (AAS) medication which was used in Europe but is now no longer marketed. It is taken by mouth.
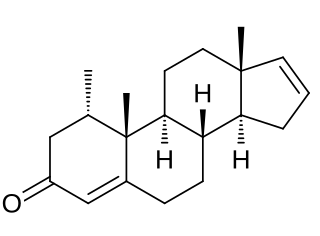
Delanterone, also known as 1α-methylandrosta-4,16-dien-3-one, is a steroidal antiandrogen described as an anti-acne agent which was never marketed. The compound showed poor efficacy as an antiandrogen in vivo in animals, suggestive of low activity or a short terminal half-life, and likely in relation to this was not further developed. It was described and characterized in the literature in 1977.

Topterone, also known as 17α-propyltestosterone or as 17α-propylandrost-4-en-17β-ol-3-one, is a steroidal antiandrogen that was first reported in 1978 and was developed for topical administration but, due to poor effectiveness, was never marketed.
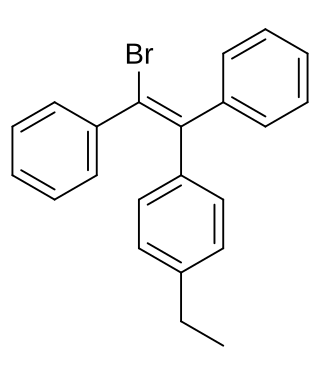
Broparestrol, also known as α-bromo-α,β-diphenyl-β-p-ethylphenylethylene (BDPE), is a synthetic, nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that has been used in Europe as a dermatological agent and for the treatment of breast cancer. The drug is described as slightly estrogenic and potently antiestrogenic, and inhibits mammary gland development and suppresses prolactin levels in animals. It is structurally related to clomifene and diethylstilbestrol. Broparestrol is a mixture of E- and Z- isomers, both of which are active, and are similarly antiestrogenic but, unlike broparestrol, were never marketed.
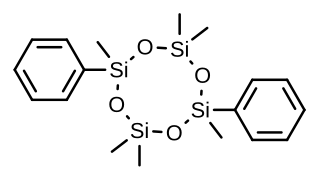
Quadrosilan is a synthetic nonsteroidal estrogen that was developed in the 1970s and that is or has been used as an antigonadotropic agent in the treatment of prostate cancer. It is an organosilicon compound, and is also known as 2,6-cisdiphenylhexamethylcyclotetrasiloxane. Quadrosilan has estrogenic activity equivalent to that of estradiol, and can produce feminization and gynecomastia as side effects in male patients.
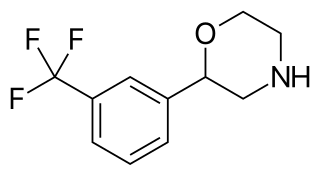
Flumexadol (INN) is a drug described and researched as a non-opioid analgesic which was never marketed. It has been found to act as an agonist of the serotonin 5-HT1A and 5-HT2C receptors and, to a much lesser extent, of the 5-HT2A receptor. According to Nilsson (2006) in a paper on 5-HT2C receptor agonists as potential anorectics, "The (+)-enantiomer of this compound showed [...] affinity for the 5-HT2C receptor (Ki) 25 nM) [...] and was 40-fold selective over the 5-HT2A receptor in receptor binding studies. Curiously, the racemic version [...], also known as 1841 CERM, was originally reported to possess analgesic properties while no association with 5-HT2C receptor activity was mentioned." It is implied that flumexadol might be employable as an anorectic in addition to analgesic. Though flumexadol itself has never been approved for medical use, oxaflozane is a prodrug of the compound that was formerly used clinically in France as an antidepressant and anxiolytic agent.

Ethinylestradiol sulfonate (EES), sold under the brand names Deposiston and Turisteron among others, is an estrogen medication which has been used in birth control pills for women and in the treatment of prostate cancer in men. It has also been investigated in the treatment of breast cancer in women. The medication was combined with norethisterone acetate in birth control pills. EES is taken by mouth once per week.
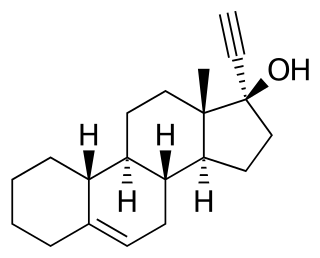
Cingestol, also known as 17α-ethynylestr-5-en-17β-ol, is a steroidal progestin of the 19-nortestosterone group that was never marketed. It was synthesized in 1969 and was developed in the 1970s by Organon as a low-dose, progestogen-only contraceptive, but in 1984, was still described as "under investigation". The drug is an isomer of lynestrenol with the double bond between C5 and C6.
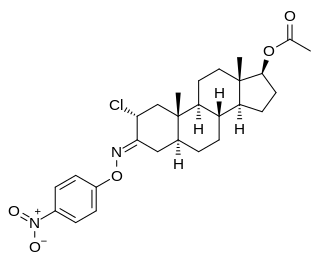
Nisterime acetate (USAN) (developmental code name ORF-9326), also known as 2α-chloro-4,5α-dihydrotestosterone O-(p-nitrophenyl)oxime 17β-acetate or as 2α-chloro-5α-androstan-17β-ol-3-one O-(p-nitrophenyl)oxime 17β-acetate, is a synthetic, orally active anabolic-androgenic steroid (AAS) and a derivative of dihydrotestosterone (DHT) that was developed as a postcoital contraceptive but was never marketed. It is an androgen ester – specifically, the C17α acetate ester of nisterime. Unlike antiprogestogens like mifepristone, nisterime acetate does not prevent implantation and instead induces embryo resorption as well as interrupts the post-implantation stage of pregnancy.
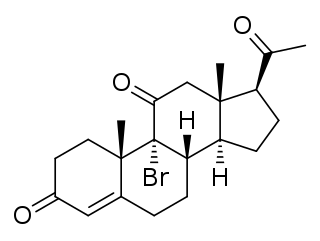
Bromoketoprogesterone (BKP), also known as 9α-bromo-11-oxoprogesterone (BOP), and known by the tentative brand name Braxarone (Squibb), is an orally active progestin which does not appear to have been marketed.





















