Related Research Articles

Viral hemorrhagic fevers (VHFs) are a diverse group of animal and human illnesses in which fever and hemorrhage are caused by a viral infection. VHFs may be caused by five distinct families of RNA viruses: the families Filoviridae, Flaviviridae, Rhabdoviridae, and several member families of the Bunyavirales order such as Arenaviridae, and Hantaviridae. All types of VHF are characterized by fever and bleeding disorders and all can progress to high fever, shock and death in many cases. Some of the VHF agents cause relatively mild illnesses, such as the Scandinavian nephropathia epidemica, while others, such as Ebola virus, can cause severe, life-threatening disease.

Crimean–Congo hemorrhagic fever (CCHF) is a viral disease. Symptoms of CCHF may include fever, muscle pains, headache, vomiting, diarrhea, and bleeding into the skin. Onset of symptoms is less than two weeks following exposure. Complications may include liver failure. In those who survive, recovery generally occurs around two weeks after onset.

Regeneron Pharmaceuticals, Inc. is an American biotechnology company headquartered in Tarrytown, New York. The company was founded in 1988. Originally focused on neurotrophic factors and their regenerative capabilities, it branched out into the study of both cytokine and tyrosine kinase receptors.

Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAb) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. More recently antibodies have been used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.
Passive immunity is the transfer of active humoral immunity of ready-made antibodies. Passive immunity can occur naturally, when maternal antibodies are transferred to the fetus through the placenta, and it can also be induced artificially, when high levels of antibodies specific to a pathogen or toxin are transferred to non-immune persons through blood products that contain antibodies, such as in immunoglobulin therapy or antiserum therapy. Passive immunization is used when there is a high risk of infection and insufficient time for the body to develop its own immune response, or to reduce the symptoms of ongoing or immunosuppressive diseases. Passive immunization can be provided when people cannot synthesize antibodies, and when they have been exposed to a disease that they do not have immunity against.
The Dale and Betty Bumpers Vaccine Research Center, more commonly known as the Vaccine Research Center (VRC), is an Intramural Division of the National Institute of Allergy and Infectious Diseases, one of the US National Institutes of Health. The mission of the VRC is "to conduct research that facilitates the development of effective vaccines for human disease." The primary focus of research is the development of vaccines for AIDS, but the VRC also is working to develop vaccines for Ebola and the Marburg virus, and therapeutic antibodies against SARS-CoV2.
A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralisation renders the particle no longer infectious or pathogenic. Neutralizing antibodies are part of the humoral response of the adaptive immune system against viruses, intracellular bacteria and microbial toxin. By binding specifically to surface structures (antigen) on an infectious particle, neutralizing antibodies prevent the particle from interacting with its host cells it might infect and destroy. Immunity due to neutralizing antibodies is also known as sterilizing immunity, as the immune system eliminates the infectious particle before any infection takes place.

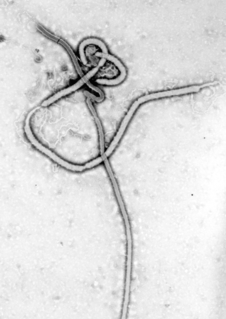
Ebola, also known as Ebola virus disease (EVD) or Ebola hemorrhagic fever (EHF), is a viral hemorrhagic fever of humans and other primates caused by ebolaviruses. Signs and symptoms typically start between two days and three weeks after contracting the virus with a fever, sore throat, muscular pain, and headaches. Vomiting, diarrhoea and rash usually follow, along with decreased function of the liver and kidneys. At this time, some people begin to bleed both internally and externally. The disease has a high risk of death, killing 25% to 90% of those infected, with an average of about 50%. This is often due to low blood pressure from fluid loss, and typically follows six to 16 days after symptoms appear.

ZMapp is an experimental biopharmaceutical drug comprising three chimeric monoclonal antibodies under development as a treatment for Ebola virus disease. Two of the three components were originally developed at the Public Health Agency of Canada's National Microbiology Laboratory (NML), and the third at the U.S. Army Medical Research Institute of Infectious Diseases; the cocktail was optimized by Gary Kobinger, a research scientist at the NML and underwent further development under license by Mapp Biopharmaceutical. ZMapp was first used on humans during the 2014 West Africa Ebola virus outbreak, having only been previously tested on animals and not yet subjected to a randomized controlled trial. The NIH ran a clinical trial starting in January 2015 with subjects from Sierra Leone, Guinea, and Liberia aiming to enroll 200 people, but the epidemic waned and the trial closed early, leaving it too statistically underpowered to give a meaningful result about whether ZMapp worked.
TKM-Ebola was an experimental antiviral drug for Ebola disease that was developed by Arbutus Biopharma in Vancouver, Canada. The drug candidate was formerly known as Ebola-SNALP.
Recombinant vesicular stomatitis virus–Zaire Ebola virus (rVSV-ZEBOV), also known as Ebola Zaire vaccine live and sold under the brand name Ervebo, is a vaccine for adults that prevents Ebola caused by the Zaire ebolavirus. When used in ring vaccination, rVSV-EBOV has shown a high level of protection. Around half the people given the vaccine have mild to moderate adverse effects that include headache, fatigue, and muscle pain.

Ebola vaccines are vaccines either approved or in development to prevent Ebola. The first vaccine to be approved in the United States was rVSV-ZEBOV in December 2019. It had been used extensively in the Kivu Ebola epidemic under a compassionate use protocol. During the early 21st century, several vaccine candidates displayed efficacy to protect nonhuman primates against lethal infection.

Galidesivir is an antiviral drug, an adenosine analog. It is developed by BioCryst Pharmaceuticals with funding from NIAID, originally intended as a treatment for hepatitis C, but subsequently developed as a potential treatment for deadly filovirus infections such as Ebola virus disease, Marburg virus disease and Zika virus. Currently, Galidesivir is under phase 1 human trial in Brazil for Coronavirus.

There is no cure or specific treatment for the Ebola virus disease that is currently approved for market, although various experimental treatments are being developed. For past and current Ebola epidemics, treatment has been primarily supportive in nature.

The 2018 Équateur province Ebola outbreak occurred in the north-west of the Democratic Republic of the Congo (DRC) from May to July 2018. It was contained entirely within Équateur province, and was the first time that vaccination with the rVSV-ZEBOV Ebola vaccine had been attempted in the early stages of an Ebola outbreak, with a total of 3,481 people vaccinated. It was the ninth recorded Ebola outbreak in the DRC.
The Kivu Ebola epidemic began on 1 August 2018, when four cases of Ebola virus disease (EVD) were confirmed in the eastern region of Kivu in the Democratic Republic of the Congo (DRC). The Kivu outbreak also affected Ituri Province, whose first case was confirmed on 13 August, and in June 2019, the virus reached Uganda, having infected a 5-year-old Congolese boy who entered Uganda with his family, but was contained. In November 2018, the outbreak became the biggest Ebola outbreak in the DRC's history, and by November, it had become the second-largest Ebola outbreak in recorded history, behind only the 2013–2016 Western Africa epidemic. On 3 May 2019, nine months into the outbreak, the DRC outbreak death toll surpassed 1,000.

Jean-Jacques Muyembe is a Congolese microbiologist. He is the General Director of the Democratic Republic of the Congo Institut National pour la Recherche Biomedicale (INRB). He was part of team at the Yambuku Catholic Mission Hospital that investigated the first Ebola outbreak, and was part of the effort that discovered Ebola as a new disease, although his exact role is still subject to controversy. In 2016, he led the research that designed, along with other researchers at the INRB and the National Institute of Health Vaccine Research Center in the US, one of the most promising treatment for Ebola, mAb114. The treatment was successfully experimented during recent outbreaks in the DRC, on the express decision of the then DRC Minister of Health, Dr Oly Ilunga, despite a prior negative advice from the World Health Organization.
REGN-EB3 is an experimental biopharmaceutical treatment comprising three monoclonal antibodies under development by Regeneron Pharmaceuticals to treat Ebola virus disease. In August 2019, Congolese health officials announced that REGN-EB3 and a similar monoclonal antibody treatment, mAb114, were more effective than two other treatments being used at the time.

Nancy Jean Sullivan is an American cell biologist researching filovirus immunology and vaccine development. She is a senior investigator and chief of the biodefense research section at the Vaccine Research Center. Her team discovered the monoclonal antibody, mAb114.
Atoltivimab/maftivimab/odesivimab, sold under the brand name Inmazeb, is a fixed-dose combination of three monoclonal antibodies for the treatment of Zaire ebolavirus. It contains atoltivimab, maftivimab, and odesivimab-ebgn.
References
- 1 2 "NIH begins testing Ebola treatment in early-stage trial". National Institutes of Health (NIH). 2018-05-23. Retrieved 2018-10-15.
- 1 2 3 4 5 6 7 8 9 10 11 12 Corti D, Misasi J, Mulangu S, Stanley DA, Kanekiyo M, Wollen S, et al. (March 2016). "Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody". Science. 351 (6279): 1339–42. Bibcode:2016Sci...351.1339C. doi: 10.1126/science.aad5224 . PMID 26917593.
- 1 2 3 Hayden EC (2016-02-26). "Ebola survivor's blood holds promise of new treatment". Nature. doi:10.1038/nature.2016.19440. ISSN 1476-4687.
- 1 2 3 4 5 6 Gaudinski MR, Coates EE, Novik L, Widge A, Houser KV, Burch E, et al. (March 2019). "Safety, tolerability, pharmacokinetics, and immunogenicity of the therapeutic monoclonal antibody mAb114 targeting Ebola virus glycoprotein (VRC 608): an open-label phase 1 study". Lancet. 393 (10174): 889–898. doi:10.1016/S0140-6736(19)30036-4. PMC 6436835 . PMID 30686586.
- 1 2 3 4 5 6 Clinical trial number NCT03478891 for "Safety and Pharmacokinetics of a Human Monoclonal Antibody, VRC-EBOMAB092-00-AB (MAb114), Administered Intravenously to Healthy Adults" at ClinicalTrials.gov
- 1 2 3 4 5 "NIH VideoCast - CC Grand Rounds: Response to an Outbreak: Ebola Virus Monoclonal Antibody (mAb114) Rapid Clinical Development". videocast.nih.gov. Retrieved 2019-08-09.
- 1 2 Kingsley-Hall A. "Congo's experimental mAb114 Ebola treatment appears successful: authorities | Central Africa". www.theafricareport.com. Retrieved 2018-10-15.
- 1 2 McNeil DG (12 August 2019). "A Cure for Ebola? Two New Treatments Prove Highly Effective in Congo". The New York Times. Retrieved 13 August 2019.
- ↑ Molteni M (12 August 2019). "Ebola is Now Curable. Here's How The New Treatments Work". Wired. Retrieved 13 August 2019.
- 1 2 Misasi J, Gilman MS, Kanekiyo M, Gui M, Cagigi A, Mulangu S, et al. (March 2016). "Structural and molecular basis for Ebola virus neutralization by protective human antibodies". Science. 351 (6279): 1343–6. Bibcode:2016Sci...351.1343M. doi:10.1126/science.aad6117. PMC 5241105 . PMID 26917592.
- ↑ Côté M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM, et al. (August 2011). "Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection". Nature. 477 (7364): 344–8. Bibcode:2011Natur.477..344C. doi:10.1038/nature10380. PMC 3230319 . PMID 21866101.
- ↑ Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, et al. (August 2011). "Ebola virus entry requires the cholesterol transporter Niemann-Pick C1". Nature. 477 (7364): 340–3. Bibcode:2011Natur.477..340C. doi:10.1038/nature10348. PMC 3175325 . PMID 21866103.
- ↑ "Ridgeback Biotherapeutics LP announces licensing of mAb114, an experimental Ebola treatment, from the National Institute of Allergy and Infectious Diseases". www.prnewswire.com. Ridgeback Biotherapeutics LP. Retrieved 2019-08-17.
- ↑ "About Us". Ridgebackbio. Retrieved 2019-08-17.
- ↑ "Ridgeback Biotherapeutics LP Announces Orphan Drug Designation for mAb114". www.prnewswire.com. Ridgeback Biotherapeutics LP. Retrieved 2019-08-17.
- ↑ Check Hayden, Erika (May 2018). "Experimental drugs poised for use in Ebola outbreak". Nature. 557 (7706): 475–476. Bibcode:2018Natur.557..475C. doi: 10.1038/d41586-018-05205-x . ISSN 0028-0836. PMID 29789732.
- ↑ WHO: Consultation on Monitored Emergency Use of Unregistered and Investigational Interventions for Ebola virus Disease. https://www.who.int/emergencies/ebola/MEURI-Ebola.pdf
- ↑ Mole, Beth (2019-08-13). "Two Ebola drugs boost survival rates, according to early trial data". Ars Technica. Retrieved 2019-08-17.
- ↑ "Independent monitoring board recommends early termination of Ebola therapeutics trial in DRC because of favorable results with two of four candidates". National Institutes of Health (NIH). 2019-08-12. Retrieved 2019-08-17.
- ↑ "FDA Approves First Treatment for Ebola Virus". U.S. Food and Drug Administration (FDA) (Press release). 14 October 2020. Retrieved 14 October 2020.
