Related Research Articles
The compound hydrogen chloride has the chemical formula HCl and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl.

Potassium chlorate is the inorganic compound with the molecular formula KClO3. In its pure form, it is a white solid. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3. It has been described as "unquestionably the most [economically] important sulfur oxide". It is prepared on an industrial scale as a precursor to sulfuric acid.

Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides.

Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K2SO4, a white water-soluble solid. It is commonly used in fertilizers, providing both potassium and sulfur.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.
The Kolbe–Schmitt reaction or Kolbe process is a carboxylation chemical reaction that proceeds by treating phenol with sodium hydroxide to form sodium phenoxide, then heating sodium phenoxide with carbon dioxide under pressure, then treating the product with sulfuric acid. The final product is an aromatic hydroxy acid which is also known as salicylic acid.

Sodium bisulfate, also known as sodium hydrogen sulfate, is the sodium salt of the bisulfate anion, with the molecular formula NaHSO4. Sodium bisulfate is an acid salt formed by partial neutralization of sulfuric acid by an equivalent of sodium base, typically in the form of either sodium hydroxide (lye) or sodium chloride (table salt). It is a dry granular product that can be safely shipped and stored. The anhydrous form is hygroscopic. Solutions of sodium bisulfate are acidic, with a 1M solution having a pH of slightly below 1.
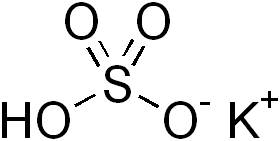
Potassium bisulfate (potassium bisulphate) is an inorganic compound with the chemical formula KHSO4 and is the potassium acid salt of sulfuric acid. It is a white, water-soluble solid.

Sodium dichloroisocyanurate is a chemical compound widely used as a cleansing agent and disinfectant. It is a colorless, water-soluble solid, produced as a result of reaction of cyanuric acid with chlorine. The dihydrate is also known as is the potassium salt.
Uranyl sulfate describes a family of inorganic compounds with the formula UO2SO4(H2O)n. These salts consist of sulfate, the uranyl ion, and water. They are lemon-yellow solids. Uranyl sulfates are intermediates in some extraction methods used for uranium ores. These compounds can also take the form of an anhydrous salt.

Chlorosulfuric acid (IUPAC name: sulfurochloridic acid) is the inorganic compound with the formula HSO3Cl. It is also known as chlorosulfonic acid, being the sulfonic acid of chlorine. It is a distillable, colorless liquid which is hygroscopic and a powerful lachrymator. Commercial samples usually are pale brown or straw colored.
In inorganic chemistry, sulfonyl halide groups occur when a sulfonyl functional group is singly bonded to a halogen atom. They have the general formula RSO2X, where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series.
Sulfation is the chemical reaction that entails the addition of SO3 group. In principle, many sulfations would involve reactions of sulfur trioxide (SO3). In practice, most sulfations are effected less directly. Regardless of the mechanism, the installation of a sulfate-like group on a substrate leads to substantial changes.

Peroxydisulfuric acid is an inorganic compound with a chemical formula (HO3SO)2. Also called Marshall's acid after Professor Hugh Marshall, who discovered it in 1891.
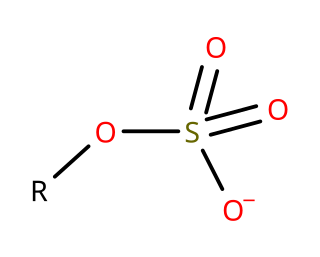
In organosulfur chemistry, organosulfates are a class of organic compounds sharing a common functional group with the structure R−O−SO−3. The SO4 core is a sulfate group and the R group is any organic residue. All organosulfates are formally esters derived from alcohols and sulfuric acid although many are not prepared in this way. Many sulfate esters are used in detergents, and some are useful reagents. Alkyl sulfates consist of a hydrophobic hydrocarbon chain, a polar sulfate group and either a cation or amine to neutralize the sulfate group. Examples include: sodium lauryl sulfate and related potassium and ammonium salts.

Ammonium bisulfate, also known as ammonium hydrogen sulfate, is a white, crystalline solid with the formula (NH4)HSO4. This salt is the product of the half-neutralization of sulfuric acid by ammonia.

Tetrakis(hydroxymethyl)phosphonium chloride (THPC) is an organophosphorus compound with the chemical formula [P(CH2OH)4]Cl. It is a white water-soluble salt. THPC has applications as a precursor to fire-retardant materials, as well as a microbiocide in commercial and industrial water systems.
Sodium pyrosulfate is an inorganic compound with the chemical formula of Na2S2O7. It is a colorless salt. It hydrolyses in water to form sodium bisulfate with a chemical formula of NaHSO4 which has a pH of around 1.

2-Nitrochlorobenzene is an organic compound with the formula ClC6H4NO2. It is one of three isomeric nitrochlorobenzenes. It is a yellow crystalline solid that is important as a precursor to other compounds due to its two functional groups.
References
- ↑ Helmold Plessen (2000). "Sodium Sulfates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_355. ISBN 978-3-527-30673-2.
- ↑ H. Schultz; G. Bauer; E. Schachl; F. Hagedorn; P. Schmittinger (2005). "Potassium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_039. ISBN 978-3-527-30673-2.
- ↑ DE 137906,"Mechanischer Calcinirofen bzw. Sulfatofen",published 1903-01-08,issued 1900-08-12
- ↑ Laury, Napoleon Arthur (1927). Hydrochloric Acid and Sodium Sulfate. Chemical catalog Company, Incorporated. ISBN 978-0-598-81795-2.
- 1 2 Riegel, Emil Raymond (1974). Kent, James Albert (ed.). Riegel's Handbook of Industrial Chemistry (7th ed.). New York: Van Nostrand Reinhold. p. 132. ISBN 978-0-442-24347-0.