Related Research Articles
RNAIII is a stable 514 nt regulatory RNA transcribed by the P3 promoter of the Staphylococcus aureus quorum-sensing agr system ). It is the major effector of the agr regulon, which controls the expression of many S. aureus genes encoding exoproteins and cell wall associated proteins plus others encoding regulatory proteins The RNAIII transcript also encodes the 26 amino acid δ-haemolysin peptide (Hld). RNAIII contains many stem loops, most of which match the Shine-Dalgarno sequence involved in translation initiation of the regulated genes. Some of these interactions are inhibitory, others stimulatory; among the former is the regulatory protein Rot. In vitro, RNAIII is expressed post exponentially, inhibiting translation of the surface proteins, notably protein A, while stimulating that of the exoproteins, many of which are tissue-degrading enzymes or cytolysins. Among the latter is the important virulence factor, α-hemolysin (Hla), whose translation RNAIII activates by preventing the formation of an inhibitory foldback loop in the hla mRNA leader.

RyhB RNA is a 90 nucleotide RNA that down-regulates a set of iron-storage and iron-using proteins when iron is limiting; it is itself negatively regulated by the ferric uptake repressor protein, Fur.

In molecular biology the ArcZ RNA is a small non-coding RNA (ncRNA). It is the functional product of a gene which is not translated into protein. ArcZ is an Hfq binding RNA that functions as an antisense regulator of a number of protein coding genes.

The Hfq protein encoded by the hfq gene was discovered in 1968 as an Escherichia coli host factor that was essential for replication of the bacteriophage Qβ. It is now clear that Hfq is an abundant bacterial RNA binding protein which has many important physiological roles that are usually mediated by interacting with Hfq binding sRNA.

Invasion gene associated RNA is a small non-coding RNA involved in regulating one of the major outer cell membrane porin proteins in Salmonella species.

An Hfq binding sRNA is an sRNA that binds the bacterial RNA binding protein called Hfq. A number of bacterial small RNAs which have been shown to bind to Hfq have been characterised . Many of these RNAs share a similar structure composed of three stem-loops. Several studies have expanded this list, and experimentally validated a total of 64 Hfq binding sRNA in Salmonella Typhimurium. A transcriptome wide study on Hfq binding sites in Salmonella mapped 126 Hfq binding sites within sRNAs. Genomic SELEX has been used to show that Hfq binding RNAs are enriched in the sequence motif 5′-AAYAAYAA-3′. Genome-wide study identified 40 candidate Hfq-dependent sRNAs in plant pathogen Erwinia amylovora. 12 of them were confirmed by Northern blot.
Sortase refers to a group of prokaryotic enzymes that modify surface proteins by recognizing and cleaving a carboxyl-terminal sorting signal. For most substrates of sortase enzymes, the recognition signal consists of the motif LPXTG (Leu-Pro-any-Thr-Gly), then a highly hydrophobic transmembrane sequence, followed by a cluster of basic residues such as arginine. Cleavage occurs between the Thr and Gly, with transient attachment through the Thr residue to the active site Cys residue, followed by transpeptidation that attaches the protein covalently to cell wall components. Sortases occur in almost all Gram-positive bacteria and the occasional Gram-negative bacterium or Archaea, where cell wall LPXTG-mediated decoration has not been reported. Although sortase A, the "housekeeping" sortase, typically acts on many protein targets, other forms of sortase recognize variant forms of the cleavage motif, or catalyze the assembly of pilins into pili.
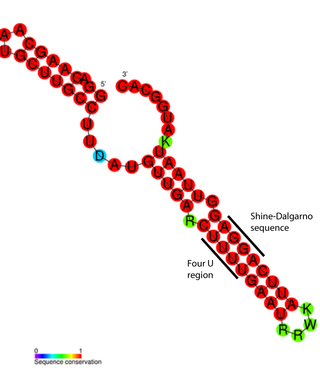
FourU thermometers are a class of non-coding RNA thermometers found in Salmonella. They are named 'FourU' due to the four highly conserved uridine nucleotides found directly opposite the Shine-Dalgarno sequence on hairpin II (pictured). RNA thermometers such as FourU control regulation of temperature via heat shock proteins in many prokaryotes. FourU thermometers are relatively small RNA molecules, only 57 nucleotides in length, and have a simple two-hairpin structure.
Bacterial small RNAs are small RNAs produced by bacteria; they are 50- to 500-nucleotide non-coding RNA molecules, highly structured and containing several stem-loops. Numerous sRNAs have been identified using both computational analysis and laboratory-based techniques such as Northern blotting, microarrays and RNA-Seq in a number of bacterial species including Escherichia coli, the model pathogen Salmonella, the nitrogen-fixing alphaproteobacterium Sinorhizobium meliloti, marine cyanobacteria, Francisella tularensis, Streptococcus pyogenes, the pathogen Staphylococcus aureus, and the plant pathogen Xanthomonas oryzae pathovar oryzae. Bacterial sRNAs affect how genes are expressed within bacterial cells via interaction with mRNA or protein, and thus can affect a variety of bacterial functions like metabolism, virulence, environmental stress response, and structure.

Streptococcal pyrogenic exotoxins also known as erythrogenic toxins, are exotoxins secreted by strains of the bacterial species Streptococcus pyogenes. SpeA and speC are superantigens, which induce inflammation by nonspecifically activating T cells and stimulating the production of inflammatory cytokines. SpeB, the most abundant streptococcal extracellular protein, is a cysteine protease. Pyrogenic exotoxins are implicated as the causative agent of scarlet fever and streptococcal toxic shock syndrome. There is no consensus on the exact number of pyrogenic exotoxins. Serotypes A, B, and C are the most extensively studied and recognized by all sources, but others note up to thirteen distinct types, categorizing speF through speM as additional superantigens. Erythrogenic toxins are known to damage the plasma membranes of blood capillaries under the skin and produce a red skin rash. Past studies have shown that multiple variants of erythrogenic toxins may be produced, depending on the strain of S. pyogenes in question. Some strains may not produce a detectable toxin at all. Bacteriophage T12 infection of S. pyogenes enables the production of speA, and increases virulence.

RivX sRNA is a non-coding RNA molecule involved in the interface between two key regulators of virulence in the human pathogen Streptococcus pyogenes : the CovR/S system and Mga regulator. This RNA, along with its downstream protein-coding gene RivR, are the first discovered links between these two important regulation networks. An extra protein linking the two pathways, TrxR, was described a year later. The adjoining of these two pathways could allow a consistently high virulence of S. pyogenes despite a variety of environmental conditions.

In molecular biology, the ferric uptake regulator family is a family of bacterial proteins involved in regulating metal ion uptake and in metal homeostasis. The family is named for its founding member, known as the ferric uptake regulator or ferric uptake regulatory protein (Fur). Fur proteins are responsible for controlling the intracellular concentration of iron in many bacteria. Iron is essential for most organisms, but its concentration must be carefully managed over a wide range of environmental conditions; high concentrations can be toxic due to the formation of reactive oxygen species.
In molecular biology, cia-dependent small RNAs (csRNAs) are small RNAs produced by Streptococci. These RNAs are part of the regulon of the CiaRH two-component regulatory system. Two of these RNAs, csRNA4 and csRNA5, have been shown to affect stationary-phase autolysis.
In molecular biology, the FasX small RNA (fibronectin/fibrinogen-binding/haemolytic-activity/streptokinase-regulator-X) is a non-coding small RNA (sRNA) produced by all group A Streptococcus. FasX has also been found in species of group D and group G Streptococcus. FasX regulates expression of secreted virulence factor streptokinase (SKA), encoded by the ska gene. FasX base pairs to the 5' end of the ska mRNA, increasing the stability of the mRNA, resulting in elevated levels of streptokinase expression. FasX negatively regulates the expression of pili and fibronectin-binding proteins on the bacterial cell surface. It binds to the 5' untranslated region of genes in the FCT-region in a serotype-specific manner, reducing the stability of and inhibiting translation of the pilus biosynthesis operon mRNA by occluding the ribosome-binding site through a simple Watson-Crick base-pairing mechanism.
In molecular biology, VR-RNA is a small RNA produced by Clostridium perfringens. It functions as a regulator of the two-component VirR/VirS system.
The gene rpoN encodes the sigma factor sigma-54, a protein in Escherichia coli and other species of bacteria. RpoN antagonizes RpoS sigma factors.
The locus of enterocyte effacement-encoded regulator (Ler) is a regulatory protein that controls bacterial pathogenicity of enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic Escherichia coli (EHEC). More specifically, Ler regulates the locus of enterocyte effacement (LEE) pathogenicity island genes, which are responsible for creating intestinal attachment and effacing lesions and subsequent diarrhea: LEE1, LEE2, and LEE3. LEE1, 2, and 3 carry the information necessary for a type III secretion system. The transcript encoding the Ler protein is the open reading frame 1 on the LEE1 operon.

RopB transcriptional regulator, also known as RopB/Rgg transcriptional regulator is a transcriptional regulator protein that regulates expression of the extracellularly secreted cysteine protease streptococcal pyrogenic exotoxin B, which is an important virulence factor of Streptococcus pyogenes and is responsible for the dissemination of a host of infectious diseases including strep throat, impetigo, streptococcal toxic shock syndrome, necrotizing fasciitis, and scarlet fever. Functional studies suggest that the ropB multigene regulon is responsible for not only global regulation of virulence but also a wide range of functions from stress response, metabolic function, and two-component signaling. Structural studies implicate ropB's regulatory action being reliant on a complex interaction involving quorum sensing with the leaderless peptide signal speB-inducing peptide (SIP) acting in conjunction with a pH sensitive histidine switch.
Parvulin-like peptidyl-prolyl isomerase (PrsA), also referred to as putative proteinase maturation protein A (PpmA), functions as a molecular chaperone in Gram-positive bacteria, such as B. subtilis, S. aureus, L. monocytogenes and S. pyogenes. PrsA proteins contain a highly conserved parvulin domain that contains peptidyl-prolyl cis-trans isomerase (PPIase) activity capable of catalyzing the bond N-terminal to proline from cis to trans, or vice versa, which is a rate limiting step in protein folding. PrsA homologs also contain a foldase domain suspected to aid in the folding of proteins but, unlike the parvulin domain, is not highly conserved. PrsA proteins are capable of forming multimers in vivo and in vitro and, when dimerized, form a claw-like structure linked by the NC domains. Most Gram-positive bacteria contain only one PrsA-like protein, but some organisms such as L. monocytogenes, B. anthracis and S. pyogenes contain two PrsAs.
Regulator gene glucosyltransferases are a family of cell signaling proteins in bacteria. Rgg proteins are part of the RRNPP superfamily of transcriptional regulators and are found in multiple Gram-positive Firmicutes bacteria, such as Streptococcus, Lactobacillus, and Listeria species. The Rgg family of proteins are quorum sensing systems that alter transcription levels by binding to DNA when the Rgg is bound to a cognate signaling Short Hydrophobic Peptide (SHP). The SHP acts as a pheromone and is generally secreted by peptidase-containing ABC transporters such as PptAB. It is thought that associated peptidases cleave the SHP into its active form upon secretion. This truncated SHP is then internalized by bacterial cells through a conserved oligopeptidase permease family. The internalized, active SHP then associates with Rgg to form a complex that binds to the promoter region of multiple genes and alters transcription. There can be several different Rgg/SHP paralogs present in a single bacterial strain, usually each with their own specific regulon. While it is theorized that each SHP can only bind to its associated Rgg, there is evidence in some species for crosstalk between different SHPs and Rggs.
References
- ↑ McIver KS, Myles RL (March 2002). "Two DNA-binding domains of Mga are required for virulence gene activation in the group A streptococcus". Molecular Microbiology. 43 (6): 1591–601. doi: 10.1046/j.1365-2958.2002.02849.x . PMID 11952907.
- ↑ Hondorp ER, McIver KS (December 2007). "The Mga virulence regulon: infection where the grass is greener". Molecular Microbiology. 66 (5): 1056–65. doi:10.1111/j.1365-2958.2007.06006.x. PMID 18001346.
- ↑ Churchward G (April 2007). "The two faces of Janus: virulence gene regulation by CovR/S in group A streptococci". Molecular Microbiology. 64 (1): 34–41. doi:10.1111/j.1365-2958.2007.05649.x. PMID 17376070.
- ↑ Roberts SA, Scott JR (December 2007). "RivR and the small RNA RivX: the missing links between the CovR regulatory cascade and the Mga regulon". Molecular Microbiology. 66 (6): 1506–22. doi:10.1111/j.1365-2958.2007.06015.x. PMID 18005100.