A mitoplast is a mitochondrion that has been stripped of its outer membrane leaving the inner membrane and matrix intact. [1]
Contents
- How mitoplasts are most commonly created
- History of mitoplast creation
- Mitoplast research
- References

A mitoplast is a mitochondrion that has been stripped of its outer membrane leaving the inner membrane and matrix intact. [1]

To begin the process, mitochondria must first be separated from cultured cells. This is typically a two step process using homogenization to release the intercellular contents and differential centrifugation to separate the mitochondria from other organelles. Once the mitochondria are isolated, mitoplasts can then be formed. Mitoplasts are most commonly formed using an apparatus called a French Press. As the mitochondria pass through the narrow valve of the French press, they experience extremely high pressures around 2,000 psi that rupture the outer mitochondrial membrane. The mitoplasts are then sedimented and kept in a specific storage buffer until use. When the mitoplasts are needed, they are simply placed in a potassium chloride (KCl) incubation buffer that causes the mitochondrial matrix to swell. As a result of the swelling, the inner membrane will protrude from the outer membrane to form one of two distinct shapes. [2] Mitoplasts generated with the French press method typically produce a bilobed vesicle and are shaped similar to a figure 8. [3] These figure 8-shaped mitoplasts are preferred because they are considered to be the healthiest. However, O-shaped mitoplasts can also form, but this type is not preferred for experimental use since they are often compromised.
The scientific understanding of mitochondria has grown tremendously since the 1930s due to development of the electron microscope. By the early 1950s, scientists were able establish to that mitochondria had two distinct membranes. However, since mitochondrial research was primarily focused on the electron transport chain and oxidative phosphorylation, it was not until over a decade later that methods were developed to separate the two mitochondrial membranes from each other.
During the mid-to-late 1960s, several independent laboratories claimed they had successfully separated the outer mitochondrial membrane and inner mitochondrial membrane. In March 1967, a team of researchers from the Nutritional Physiology Laboratory in France published an article that discussed their studies of the enzymatic activities of the outer mitochondrial membrane. Since their studies required the isolation of the outer mitochondrial membrane, a method based on the successive actions of digitonin and sonication for separating the two mitochondrial membranes was also described. To complete their isolation procedure, the crude fraction of the outer membrane was purified using differential centrifugation. [4] About a year later in July 1968, a research team from the Department of Physiological Chemistry at Johns Hopkins School of Medicine published an article describing their method for separation of the mitochondrial membranes in rat livers also using digitonin and differential centrifugation. In addition to separating the outer and inner membranes, they were able to further separate the inner membrane and matrix through treatment with a nonionic detergent called Lubrol. This process then allowed for calculation of the relative protein content within each mitochondrial component. [5] The rationale amongst both articles for using low concentrations of digitonin to detach the outer mitochondrial membrane was that the outer membrane was rich in cholesterol which would cause it to bind to the digitonin. After further study, the research team from Johns Hopkins was able to refine their method of mitoplast preparation to produce mitochondria without an outer membrane and with a relatively intact inner membrane and matrix.
Another procedure for mitoplast preparation, described by two additional research groups, took advantage of the selective shrinking of the inner membrane after liver mitochondria were exposed to a swelling-contraction cycle. During this procedure, the swollen outer membrane ruptured either spontaneously or ideally after being subjected to gentle sonication. Following the rupture of the outer membrane, the inner membrane components could be separated from the outer membrane by differential centrifugation. [6] This procedure is effective due to the distinct differences between the outer and inner mitochondrial membranes, specifically those regarding osmotic behavior and permeability. According to extensive research, the inner membrane is able to respond to changes in osmotic pressure by unfolding and refolding. However, the outer membrane has shown no reversible responses to changes in osmotic pressure. Therefore, the distention and rupture of the outer membrane is a passive process caused by unfolding of the inner membrane due to change in osmotic pressure.
Although these methods of separation were proven to be fairly efficient at the time, their mechanical nature became outdated as new technology, such as the French Press, was developed to make mitoplast creation quicker and easier for researchers .
The ability for researchers to separate the two mitochondrial membranes to form a mitoplast has created many new possibilities for mitochondrion studies. As mentioned previously, researchers have now been able to calculate the relative protein content within each component of a mitochondrion. In addition, mitoplasts enabled the determination of the intramitochondrial distribution of enzymes, which had previously been impossible since the inner membrane was encased by the outer membrane. Therefore, not long after mitoplasts could be readily created, various data became available regarding almost all enzymes that had been suspected to be present in mitochondria.

Mitoplasts are also useful for electrophysiological analysis of mitochondrial function since mitochondria can retain normal function even after their outer membrane has been removed. Specifically, patch-clamp electrophysiology has emerged as a novel method for studying functionality of the inner membrane. [7] This method facilitates sensitive current measurement across the membrane, useful for studying the electron transport chain and proton leak (i.e., the uncoupling of proton movement down its electrochemical gradient and ATP synthesis via ATP synthase).

A mitochondrion is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term mitochondrion was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name.
Protein targeting or protein sorting is the biological mechanism by which proteins are transported to their appropriate destinations within or outside the cell. Proteins can be targeted to the inner space of an organelle, different intracellular membranes, the plasma membrane, or to the exterior of the cell via secretion. Information contained in the protein itself directs this delivery process. Correct sorting is crucial for the cell; errors or dysfunction in sorting have been linked to multiple diseases.

In cell biology, a vesicle is a structure within or outside a cell, consisting of liquid or cytoplasm enclosed by a lipid bilayer. Vesicles form naturally during the processes of secretion (exocytosis), uptake (endocytosis), and the transport of materials within the plasma membrane. Alternatively, they may be prepared artificially, in which case they are called liposomes. If there is only one phospholipid bilayer, the vesicles are called unilamellar liposomes; otherwise they are called multilamellar liposomes. The membrane enclosing the vesicle is also a lamellar phase, similar to that of the plasma membrane, and intracellular vesicles can fuse with the plasma membrane to release their contents outside the cell. Vesicles can also fuse with other organelles within the cell. A vesicle released from the cell is known as an extracellular vesicle.
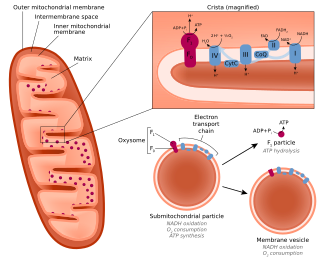
A submitochondrial particle (SMP) is an artificial vesicle made from the inner mitochondrial membrane. They can be formed by subjecting isolated mitochondria to sonication, freezing and thawing, high pressure, or osmotic shock. SMPs can be used to study the electron transport chain in a cell-free context.

A crista is a fold in the inner membrane of a mitochondrion. The name is from the Latin for crest or plume, and it gives the inner membrane its characteristic wrinkled shape, providing a large amount of surface area for chemical reactions to occur on. This aids aerobic cellular respiration, because the mitochondrion requires oxygen. Cristae are studded with proteins, including ATP synthase and a variety of cytochromes.

Chemiosmosis is the movement of ions across a semipermeable membrane bound structure, down their electrochemical gradient. An important example is the formation of adenosine triphosphate (ATP) by the movement of hydrogen ions (H+) across a membrane during cellular respiration or photosynthesis.
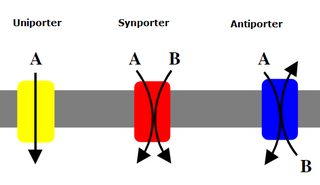
Uniporters, also known as solute carriers or facilitated transporters, are a type of membrane transport protein that passively transports solutes across a cell membrane. It uses facilitated diffusion for the movement of solutes down their concentration gradient from an area of high concentration to an area of low concentration. Unlike active transport, it does not require energy in the form of ATP to function. Uniporters are specialized to carry one specific ion or molecule and can be categorized as either channels or carriers. Facilitated diffusion may occur through three mechanisms: uniport, symport, or antiport. The difference between each mechanism depends on the direction of transport, in which uniport is the only transport not coupled to the transport of another solute.

In the mitochondrion, the matrix is the space within the inner membrane. The word "matrix" stems from the fact that this space is viscous, compared to the relatively aqueous cytoplasm. The mitochondrial matrix contains the mitochondrial DNA, ribosomes, soluble enzymes, small organic molecules, nucleotide cofactors, and inorganic ions.[1] The enzymes in the matrix facilitate reactions responsible for the production of ATP, such as the citric acid cycle, oxidative phosphorylation, oxidation of pyruvate, and the beta oxidation of fatty acids.

The intermembrane space (IMS) is the space occurring between or involving two or more membranes. In cell biology, it is most commonly described as the region between the inner membrane and the outer membrane of a mitochondrion or a chloroplast. It also refers to the space between the inner and outer nuclear membranes of the nuclear envelope, but is often called the perinuclear space. The IMS of mitochondria plays a crucial role in coordinating a variety of cellular activities, such as regulation of respiration and metabolic functions. Unlike the IMS of the mitochondria, the IMS of the chloroplast does not seem to have any obvious function.
Cardiolipin is an important component of the inner mitochondrial membrane, where it constitutes about 20% of the total lipid composition. It can also be found in the membranes of most bacteria. The name "cardiolipin" is derived from the fact that it was first found in animal hearts. It was first isolated from the beef heart in the early 1940s by Mary C. Pangborn. In mammalian cells, but also in plant cells, cardiolipin (CL) is found almost exclusively in the inner mitochondrial membrane, where it is essential for the optimal function of numerous enzymes that are involved in mitochondrial energy metabolism.

The inner mitochondrial membrane (IMM) is the mitochondrial membrane which separates the mitochondrial matrix from the intermembrane space.
The mitochondrial permeability transition pore is a protein that is formed in the inner membrane of the mitochondria under certain pathological conditions such as traumatic brain injury and stroke. Opening allows increase in the permeability of the mitochondrial membranes to molecules of less than 1500 daltons in molecular weight. Induction of the permeability transition pore, mitochondrial membrane permeability transition, can lead to mitochondrial swelling and cell death through apoptosis or necrosis depending on the particular biological setting.

Mitochondrial membrane transport proteins, also known as mitochondrial carrier proteins, are proteins which exist in the membranes of mitochondria. They serve to transport molecules and other factors, such as ions, into or out of the organelles. Mitochondria contain both an inner and outer membrane, separated by the inter-membrane space, or inner boundary membrane. The outer membrane is porous, whereas the inner membrane restricts the movement of all molecules. The two membranes also vary in membrane potential and pH. These factors play a role in the function of mitochondrial membrane transport proteins. There are 53 discovered human mitochondrial membrane transporters, with many others that are known to still need discovered.

Adenine nucleotide translocator (ANT), also known as the ADP/ATP translocase (ANT), ADP/ATP carrier protein (AAC) or mitochondrial ADP/ATP carrier, exchanges free ATP with free ADP across the inner mitochondrial membrane. ANT is the most abundant protein in the inner mitochondrial membrane and belongs to mitochondrial carrier family.

Glycerol-3-phosphate dehydrogenase (GPDH) is an enzyme that catalyzes the reversible redox conversion of dihydroxyacetone phosphate to sn-glycerol 3-phosphate.

The translocase of the outer membrane (TOM) is a complex of proteins found in the outer mitochondrial membrane of the mitochondria. It allows movement of proteins through this barrier and into the intermembrane space of the mitochondrion. Most of the proteins needed for mitochondrial function are encoded by the nucleus of the cell. The outer membrane of the mitochondrion is impermeable to large molecules greater than 5000 daltons. The TOM works in conjunction with the translocase of the inner membrane (TIM) to translocate proteins into the mitochondrion. Many of the proteins in the TOM complex, such as TOMM22, were first identified in Neurospora crassa and Saccharomyces cerevisiae. Many of the genes encoding these proteins are designated as TOMM (translocase of the outer mitochondrial membrane) complex genes.
Mitochondrial biogenesis is the process by which cells increase mitochondrial numbers. It was first described by John Holloszy in the 1960s, when it was discovered that physical endurance training induced higher mitochondrial content levels, leading to greater glucose uptake by muscles. Mitochondrial biogenesis is activated by numerous different signals during times of cellular stress or in response to environmental stimuli, such as aerobic exercise.
Mitochondria are dynamic organelles with the ability to fuse and divide (fission), forming constantly changing tubular networks in most eukaryotic cells. These mitochondrial dynamics, first observed over a hundred years ago are important for the health of the cell, and defects in dynamics lead to genetic disorders. Through fusion, mitochondria can overcome the dangerous consequences of genetic malfunction. The process of mitochondrial fusion involves a variety of proteins that assist the cell throughout the series of events that form this process.
The mitochondrial calcium uniporter (MCU) is a transmembrane protein that allows the passage of calcium ions from a cell's cytosol into mitochondria. Its activity is regulated by MICU1 and MICU2, which together with the MCU make up the mitochondrial calcium uniporter complex.

Mitochondria-associated membranes (MAM) represent a region of the endoplasmic reticulum (ER) which is reversibly tethered to mitochondria. These membranes are involved in import of certain lipids from the ER to mitochondria and in regulation of calcium homeostasis, mitochondrial function, autophagy and apoptosis. They also play a role in development of neurodegenerative diseases and glucose homeostasis.