
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.

The troposphere is the first and lowest layer of the atmosphere of the Earth, and contains 75% of the total mass of the planetary atmosphere, 99% of the total mass of water vapour and aerosols, and is where most weather phenomena occur. From the planetary surface of the Earth, the average height of the troposphere is 18 km in the tropics; 17 km in the middle latitudes; and 6 km in the high latitudes of the polar regions in winter; thus the average height of the troposphere is 13 km.

The stratosphere is the second layer of the atmosphere of the Earth, located above the troposphere and below the mesosphere. The stratosphere is an atmospheric layer composed of stratified temperature layers, with the warm layers of air high in the sky and the cool layers of air in the low sky, close to the planetary surface of the Earth. The increase of temperature with altitude is a result of the absorption of the Sun's ultraviolet (UV) radiation by the ozone layer. The temperature inversion is in contrast to the troposphere, near the Earth's surface, where temperature decreases with altitude.
The thermosphere is the layer in the Earth's atmosphere directly above the mesosphere and below the exosphere. Within this layer of the atmosphere, ultraviolet radiation causes photoionization/photodissociation of molecules, creating ions; the thermosphere thus constitutes the larger part of the ionosphere. Taking its name from the Greek θερμός meaning heat, the thermosphere begins at about 80 km (50 mi) above sea level. At these high altitudes, the residual atmospheric gases sort into strata according to molecular mass. Thermospheric temperatures increase with altitude due to absorption of highly energetic solar radiation. Temperatures are highly dependent on solar activity, and can rise to 2,000 °C (3,630 °F) or more. Radiation causes the atmospheric particles in this layer to become electrically charged, enabling radio waves to be refracted and thus be received beyond the horizon. In the exosphere, beginning at about 600 km (375 mi) above sea level, the atmosphere turns into space, although, by the judging criteria set for the definition of the Kármán line (100 km), most of the thermosphere is part of space. The border between the thermosphere and exosphere is known as the thermopause.
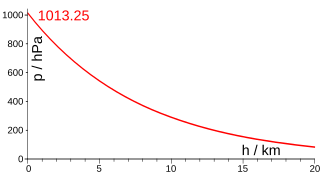
Atmospheric pressure, also known as barometric pressure, is the pressure within the atmosphere of Earth. The standard atmosphere is a unit of pressure defined as 101,325 Pa (1,013.25 hPa), which is equivalent to 1013.25 millibars, 760 mm Hg, 29.9212 inches Hg, or 14.696 psi. The atm unit is roughly equivalent to the mean sea-level atmospheric pressure on Earth; that is, the Earth's atmospheric pressure at sea level is approximately 1 atm.

A barometer is a scientific instrument that is used to measure air pressure in a certain environment. Pressure tendency can forecast short term changes in the weather. Many measurements of air pressure are used within surface weather analysis to help find surface troughs, pressure systems and frontal boundaries.
The molar gas constant is denoted by the symbol R or R. It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per amount of substance, i.e. the pressure–volume product, rather than energy per temperature increment per particle. The constant is also a combination of the constants from Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. It is a physical constant that is featured in many fundamental equations in the physical sciences, such as the ideal gas law, the Arrhenius equation, and the Nernst equation.

The speed of sound is the distance travelled per unit of time by a sound wave as it propagates through an elastic medium. At 20 °C (68 °F), the speed of sound in air is about 343 metres per second, or one kilometre in 2.91 s or one mile in 4.69 s. It depends strongly on temperature as well as the medium through which a sound wave is propagating. At 0 °C (32 °F), the speed of sound in air is about 331 m/s.

The atmosphere of Earth or air is the layer of gases retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing for liquid water to exist on the Earth's surface, absorbing ultraviolet solar radiation, warming the surface through heat retention, and reducing temperature extremes between day and night.

An atmosphere is a layer of gas or layers of gases that envelope a planet, and is held in place by the gravity of the planetary body. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosphere is the outer region of a star, which includes the layers above the opaque photosphere; stars of low temperature might have outer atmospheres containing compound molecules.

Fluid statics or hydrostatics is the branch of fluid mechanics that studies the condition of the equilibrium of a floating body and submerged body "fluids at hydrostatic equilibrium and the pressure in a fluid, or exerted by a fluid, on an immersed body".
The Kármán line is an attempt to define a boundary between Earth's atmosphere and outer space, and offers a specific definition set by the Fédération aéronautique internationale (FAI), an international record-keeping body for aeronautics. Defining the edge of space is important for legal and regulatory purposes since aircraft and spacecraft fall under different jurisdictions and are subject to different treaties. International law does not define the edge of space, or the limit of national airspace.
In atmospheric, earth, and planetary sciences, a scale height, usually denoted by the capital letter H, is a distance over which a physical quantity decreases by a factor of e.

The U.S. Standard Atmosphere is a static atmospheric model of how the pressure, temperature, density, and viscosity of the Earth's atmosphere change over a wide range of altitudes or elevations. The model, based on an existing international standard, was first published in 1958 by the U.S. Committee on Extension to the Standard Atmosphere, and was updated in 1962, 1966, and 1976. It is largely consistent in methodology with the International Standard Atmosphere, differing mainly in the assumed temperature distribution at higher altitudes.
A reference atmospheric model describes how the ideal gas properties of an atmosphere change, primarily as a function of altitude, and sometimes also as a function of latitude, day of year, etc. A static atmospheric model has a more limited domain, excluding time. A standard atmosphere is defined by the World Meteorological Organization as "a hypothetical vertical distribution of atmospheric temperature, pressure and density which, by international agreement, is roughly representative of year-round, midlatitude conditions."
In common usage, the mass of an object is often referred to as its weight, though these are in fact different concepts and quantities. Nevertheless, one object will always weigh more than a second object, if the first object has greater mass, and the two objects are subject to the same gravity.

The degree Celsius is the unit of temperature on the Celsius scale,, one of 2 temperature scales used in the International System of Units (SI), alongside the Kelvin scale. The degree Celsius can refer to a specific temperature on the Celsius scale or a unit to indicate a difference or range between two temperatures. It is named after the Swedish astronomer Anders Celsius (1701–1744), who developed a similar temperature scale in 1742. Before being renamed to honour Anders Celsius in 1948, the unit was called centigrade, from the Latin centum, which means 100, and gradus, which means steps. Most major countries use this scale; the other major scale, Fahrenheit, is still used in the United States, some island territories, and Liberia. The Kelvin scale is of use in the sciences, with 0 K (−273.15 °C) representing absolute zero.

The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow based engineer and physicist William Thomson, 1st Baron Kelvin (1824–1907). The Kelvin scale is an absolute thermodynamic temperature scale, meaning it uses absolute zero as its null (zero) point.

Temperature is a physical quantity that expresses the hotness of matter or radiation.
Vertical pressure variation is the variation in pressure as a function of elevation. Depending on the fluid in question and the context being referred to, it may also vary significantly in dimensions perpendicular to elevation as well, and these variations have relevance in the context of pressure gradient force and its effects. However, the vertical variation is especially significant, as it results from the pull of gravity on the fluid; namely, for the same given fluid, a decrease in elevation within it corresponds to a taller column of fluid weighing down on that point.













