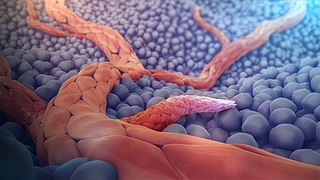
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature mainly by processes of sprouting and splitting, but processes such as coalescent angiogenesis, vessel elongation and vessel cooption also play a role. Vasculogenesis is the embryonic formation of endothelial cells from mesoderm cell precursors, and from neovascularization, although discussions are not always precise. The first vessels in the developing embryo form through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth during development and in disease.

The endothelium is a single layer of squamous endothelial cells that line the interior surface of blood vessels and lymphatic vessels. The endothelium forms an interface between circulating blood or lymph in the lumen and the rest of the vessel wall.

Platelet-derived growth factor (PDGF) is one among numerous growth factors that regulate cell growth and division. In particular, PDGF plays a significant role in blood vessel formation, the growth of blood vessels from already-existing blood vessel tissue, mitogenesis, i.e. proliferation, of mesenchymal cells such as fibroblasts, osteoblasts, tenocytes, vascular smooth muscle cells and mesenchymal stem cells as well as chemotaxis, the directed migration, of mesenchymal cells. Platelet-derived growth factor is a dimeric glycoprotein that can be composed of two A subunits (PDGF-AA), two B subunits (PDGF-BB), or one of each (PDGF-AB).
Vascular endothelial growth factor, originally known as vascular permeability factor (VPF), is a signal protein produced by many cells that stimulates the formation of blood vessels. To be specific, VEGF is a sub-family of growth factors, the platelet-derived growth factor family of cystine-knot growth factors. They are important signaling proteins involved in both vasculogenesis and angiogenesis.
An angiogenesis inhibitor is a substance that inhibits the growth of new blood vessels (angiogenesis). Some angiogenesis inhibitors are endogenous and a normal part of the body's control and others are obtained exogenously through pharmaceutical drugs or diet.

Endostatin is a naturally occurring, 20-kDa C-terminal fragment derived from type XVIII collagen. It is reported to serve as an anti-angiogenic agent, similar to angiostatin and thrombospondin.
Endothelial stem cells (ESCs) are one of three types of stem cells found in bone marrow. They are multipotent, which describes the ability to give rise to many cell types, whereas a pluripotent stem cell can give rise to all types. ESCs have the characteristic properties of a stem cell: self-renewal and differentiation. These parent stem cells, ESCs, give rise to progenitor cells, which are intermediate stem cells that lose potency. Progenitor stem cells are committed to differentiating along a particular cell developmental pathway. ESCs will eventually produce endothelial cells (ECs), which create the thin-walled endothelium that lines the inner surface of blood vessels and lymphatic vessels. The blood vessels include arteries and veins. Endothelial cells can be found throughout the whole vascular system and they also play a vital role in the movement of white blood cells

Angiopoietin is part of a family of vascular growth factors that play a role in embryonic and postnatal angiogenesis. Angiopoietin signaling most directly corresponds with angiogenesis, the process by which new arteries and veins form from preexisting blood vessels. Angiogenesis proceeds through sprouting, endothelial cell migration, proliferation, and vessel destabilization and stabilization. They are responsible for assembling and disassembling the endothelial lining of blood vessels. Angiopoietin cytokines are involved with controlling microvascular permeability, vasodilation, and vasoconstriction by signaling smooth muscle cells surrounding vessels. There are now four identified angiopoietins: ANGPT1, ANGPT2, ANGPTL3, ANGPT4.

Thrombospondin 1, abbreviated as THBS1, is a protein that in humans is encoded by the THBS1 gene.

VEGF receptors (VEGFRs) are receptors for vascular endothelial growth factor (VEGF). There are three main subtypes of VEGFR, numbered 1, 2 and 3. Depending on alternative splicing, they may be membrane-bound (mbVEGFR) or soluble (sVEGFR).

Vascular endothelial growth factor receptor 1 is a protein that in humans is encoded by the FLT1 gene.

Thymidine phosphorylase is an enzyme that is encoded by the TYMP gene and catalyzes the reaction:

Pigment epithelium-derived factor (PEDF) also known as serpin F1 (SERPINF1), is a multifunctional secreted protein that has anti-angiogenic, anti-tumorigenic, and neurotrophic functions. Found in vertebrates, this 50 kDa protein is being researched as a therapeutic candidate for treatment of such conditions as choroidal neovascularization, heart disease, and cancer. In humans, pigment epithelium-derived factor is encoded by the SERPINF1 gene.

Placental growth factor(PlGF) is a protein that in humans is encoded by the PGF gene.

C-fos-induced growth factor (FIGF) is a vascular endothelial growth factor that in humans is encoded by the FIGF gene.
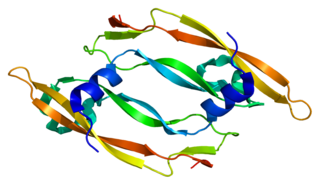
Vascular endothelial growth factor B also known as VEGF-B is a protein that, in humans, is encoded by the VEGF-B gene. VEGF-B is a growth factor that belongs to the vascular endothelial growth factor family, of which VEGF-A is the best-known member.

Vascular endothelial growth factor A (VEGF-A) is a protein that in humans is encoded by the VEGFA gene.
Angiogenesis is the process of forming new blood vessels from existing blood vessels, formed in vasculogenesis. It is a highly complex process involving extensive interplay between cells, soluble factors, and the extracellular matrix (ECM). Angiogenesis is critical during normal physiological development, but it also occurs in adults during inflammation, wound healing, ischemia, and in pathological conditions such as rheumatoid arthritis, hemangioma, and tumor growth. Proteolysis has been indicated as one of the first and most sustained activities involved in the formation of new blood vessels. Numerous proteases including matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase domain (ADAM), a disintegrin and metalloproteinase domain with throbospondin motifs (ADAMTS), and cysteine and serine proteases are involved in angiogenesis. This article focuses on the important and diverse roles that these proteases play in the regulation of angiogenesis.

Vasculogenic mimicry (VM) is a strategy used by tumors to ensure sufficient blood supply is brought to its cells through establishing new tumor vascularization. This process is similar to tumor angiogenesis; on the other hand vascular mimicry is unique in that this process occurs independent of endothelial cells. Vasculature is instead developed de novo by cancer cells, which under stress conditions such as hypoxia, express similar properties to stem cells, capable of differentiating to mimic the function of endothelial cells and form vasculature-like structures. The ability of tumors to develop and harness nearby vasculature is considered one of the hallmarks of cancer disease development and is thought to be closely linked to tumor invasion and metastasis. Vascular mimicry has been observed predominantly in aggressive and metastatic cancers and has been associated with negative tumor characteristics such as increased metastasis, increased tissue invasion, and overall poor outcomes for patient survival. Vascular mimicry poses a serious problem for current therapeutic strategies due to its ability to function in the presence of Anti-angiogenic therapeutic agents. In fact, such therapeutics have been found to actually drive VM formation in tumors, causing more aggressive and difficult to treat tumors to develop.

Endothelial cell anergy is a condition during the process of angiogenesis, where endothelial cells, the cells that line the inside of blood vessels, can no longer respond to inflammatory cytokines. These cytokines are necessary to induce the expression of cell adhesion molecules to allow leukocyte infiltration from the blood into the tissue at places of inflammation, such as a tumor. This condition, which protects the tumor from the immune system, is the result of exposure to angiogenic growth factors.
















