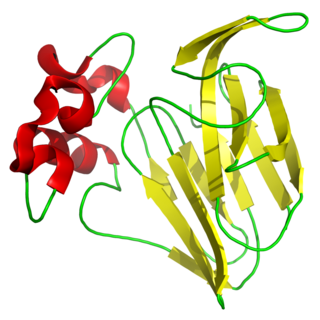
Thaumatin is a low-calorie sweetener and flavor modifier. The protein is often used primarily for its flavor-modifying properties and not exclusively as a sweetener.

Acesulfame potassium, also known as acesulfame K or Ace K, is a synthetic calorie-free sugar substitute often marketed under the trade names Sunett and Sweet One. In the European Union, it is known under the E number E950. It was discovered accidentally in 1967 by German chemist Karl Clauss at Hoechst AG. In chemical structure, acesulfame potassium is the potassium salt of 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide. It is a white crystalline powder with molecular formula C
4H
4KNO
4S and a molecular weight of 201.24 g/mol.

UniProt is a freely accessible database of protein sequence and functional information, many entries being derived from genome sequencing projects. It contains a large amount of information about the biological function of proteins derived from the research literature. It is maintained by the UniProt consortium, which consists of several European bioinformatics organisations and a foundation from Washington, DC, USA.
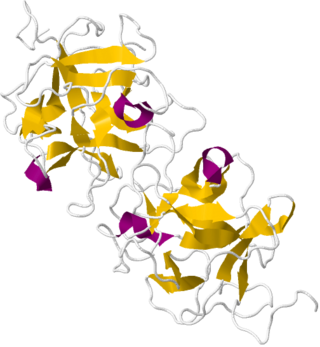
Miraculin is a taste modifier, a glycoprotein extracted from the fruit of Synsepalum dulcificum. The berry, also known as the miracle fruit, was documented by explorer Chevalier des Marchais, who searched for many different fruits during a 1725 excursion to its native West Africa.

Lactisole is the sodium salt and commonly supplied form of 2-(4-methoxyphenoxy)propionic acid, a natural carboxylic acid found in roasted coffee beans. Like gymnemic acid, it has the property of masking sweet flavors and is used for this purpose in the food industry.

Sweetness is a basic taste most commonly perceived when eating foods rich in sugars. Sweet tastes are generally regarded as pleasurable. In addition to sugars like sucrose, many other chemical compounds are sweet, including aldehydes, ketones, and sugar alcohols. Some are sweet at very low concentrations, allowing their use as non-caloric sugar substitutes. Such non-sugar sweeteners include saccharin, aspartame, sucralose and stevia. Other compounds, such as miraculin, may alter perception of sweetness itself.
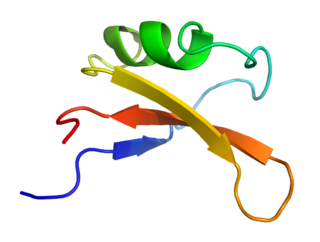
Brazzein is a protein found in the West African fruit of Oubli. It was first isolated by the University of Wisconsin–Madison in 1994.

Perillartine, also known as perillartin and perilla sugar, is a semisynthetic sweetener that is about 2000 times as sweet as sucrose. It is mainly used in Japan. Perillartine is the oxime of perillaldehyde, which is extracted from plants of the genus Perilla (Lamiaceae).

CSF2RB is a common subunit to the following type I cytokine receptors:
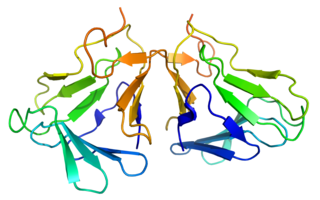
Curculin or neoculin is a sweet protein that was discovered and isolated in 1990 from the fruit of Curculigo latifolia (Hypoxidaceae), a plant from Malaysia. Like miraculin, curculin exhibits taste-modifying activity; however, unlike miraculin, it also exhibits a sweet taste by itself. After consumption of curculin, water and sour solutions taste sweet. The plant is referred to locally as 'Lumbah' or 'Lemba'.
Gymnemic acids are a class of chemical compounds isolated from the leaves of Gymnema sylvestre (Asclepiadaceae). They are anti-sweet compounds, or sweetness inhibitors. After chewing the leaves, solutions sweetened with sugar taste like water.

Pentadin, a sweet-tasting protein, was discovered and isolated in 1989, in the fruit of oubli, a climbing shrub growing in some tropical countries of Africa. Sweet tasting proteins are often used in the treatment of diabetes, obesity, and other metabolic disorders that one can experience. These proteins are isolated from the pulp of various fruits, typically found in rain forests and are also used as low calorie sweeteners that can enhance and modify existing foods.

Mabinlins are sweet-tasting proteins extracted from the seed of mabinlang, a plant growing in Yunnan province of China. There are four homologues. Mabinlin-2 was first isolated in 1983 and characterised in 1993, and is the most extensively studied of the four. The other variants of mabinlin-1, -3 and -4 were discovered and characterised in 1994.

Hemoglobin subunit gamma-2 is a protein that in humans is encoded by the HBG2 gene.

T1R2 - Taste receptor type 1 member 2 is a protein that in humans is encoded by the TAS1R2 gene.

Succinyl-CoA ligase [GDP-forming] subunit alpha, mitochondrial is an enzyme that in humans is encoded by the SUCLG1 gene.

Thaumatococcus daniellii, also known as miracle fruit or miracle berry, is a plant species from tropical Africa of the Marantaceae family. It is a large, rhizomatous, flowering herb native to the rainforests of western Africa in Sierra Leone, southeast to Gabon and the Democratic Republic of the Congo. It is also an introduced species in Australia and Singapore.
Suosan is calorie-free artificial sweetener derived from β-alanine, discovered in 1948 by Petersen et Muller.

Carrelame is an extremely high potency artificial sweetener of the guanidine class, closely related to lugduname. While Carrelame is roughly 200,000 times as sweet as sucrose, lugduname is still somewhat sweeter. It appears safe in pigs.
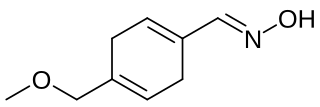
Oxime V is a chemical compound that has been studied as a potential sweetener. Oxime V was first reported in 1976 as a synthetic analog of the artificial sweetener perillartine. It is about 450 times as sweet as sucrose and is more water-soluble than perillartine. Its metabolism and toxicology have been investigated, and it has been found to have promising properties, but it is not currently marketed.


















