
A steroid is an organic compound with four fused rings arranged in a specific molecular configuration.

Batrachotoxin (BTX) is an extremely potent cardio- and neurotoxic steroidal alkaloid found in certain species of beetles, birds, and frogs. The name is from the Greek word βάτραχος, bátrachos, 'frog'. Structurally-related chemical compounds are often referred to collectively as batrachotoxins. In certain frogs, this alkaloid is present mostly on the skin. Such frogs are among those used for poisoning darts. Batrachotoxin binds to and irreversibly opens the sodium channels of nerve cells and prevents them from closing, resulting in paralysis and death. No antidote is known.

Veratrum viride, known as Indian poke, corn-lily, Indian hellebore, false hellebore, green false hellebore, or giant false-helleborine, is a species of Veratrum native to eastern and western North America. It is extremely toxic, and is considered a pest plant by farmers with livestock. The species has acquired a large number of other common names within its native range, including American false hellebore, American white hellebore, bear corn, big hellebore, corn lily, devil's bite, duck retten, itchweed, poor Annie, blue hellebore and tickleweed.

Cyclopamine (11-deoxojervine) is a naturally occurring steroidal alkaloid. It is a teratogenic component of corn lily, which when consumed during gestation has been demonstrated to induce birth defects, including the development of a single eye (cyclopia) in offspring. The molecule was named after this effect, which was originally observed by Idaho lamb farmers in 1957 after their herds gave birth to cycloptic lambs. It then took more than a decade to identify corn lily as the culprit. Later work suggested that differing rain patterns had changed grazing behaviours, which led to a greater quantity of corn lily to be ingested by pregnant sheep. Cyclopamine interrupts the sonic hedgehog signalling pathway, instrumental in early development, ultimately causing birth defects.

Veratridine is a steroidal alkaloid found in plants of the lily family, specifically the genera Veratrum and Schoenocaulon. Upon absorption through the skin or mucous membranes, it acts as a neurotoxin by binding to and preventing the inactivation of voltage-gated sodium ion channels in heart, nerve, and skeletal muscle cell membranes. Veratridine increases nerve excitability and intracellular Ca2+ concentrations.
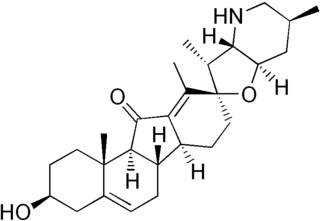
Jervine is a steroidal alkaloid with molecular formula C27H39NO3 which is derived from the plant genus Veratrum. Similar to cyclopamine, which also occurs in the genus Veratrum, it is a teratogen implicated in birth defects when consumed by animals during a certain period of their gestation.

Steroid sulfatase (STS), or steryl-sulfatase, formerly known as arylsulfatase C, is a sulfatase enzyme involved in the metabolism of steroids. It is encoded by the STS gene.

Veratrum is a genus of flowering plants in the family Melanthiaceae. It occurs in damp habitats across much of temperate and subarctic Europe, Asia, and North America.

Veratrum album, the false helleborine, white hellebore, European white hellebore, or white veratrum is a poisonous plant in the family Melanthiaceae. It is native to Europe and parts of western Asia.

Veratrum californicum is an extremely poisonous plant native to western North America, including the Sierra Nevada and Rocky Mountains, as far north as Washington and as far south as Durango; depending on latitude, it grows from near sea level to as high as 11,000 feet. It grows 1 to 2 meters tall, with an erect, unbranched, heavily leafy stem resembling a cornstalk. It prefers quite moist soil, and can cover large areas in dense stands near streams or in wet meadows. Many inch-wide flowers cluster along the often-branched top of the stout stem; they have 6 white tepals, a green center, 6 stamens, and a 3-branched pistil. The buds are tight green spheres. The heavily veined, bright green leaves can be more than a foot long.

Glaucine(1,2,9,10-TetraMethoxyAporphine, Bromcholitin, Glauvent, Tusidil, Tussiglaucin) is an aporphine alkaloid found in several different plant species in the family Papaveraceae such as Glaucium flavum, Glaucium oxylobum and Corydalis yanhusuo, and in other plants like Croton lechleri in the family Euphorbiaceae.

Conessine is a steroidal alkaloid found in a number of plant species from the family Apocynaceae, including Holarrhena floribunda, Holarrhena antidysenterica and Funtumia elastica. It acts as a histamine antagonist, selective for the H3 subtype (with an affinity of pKi = 8.27; Ki = ~5 nM). It was also found to have long CNS clearance times, high blood–brain barrier penetration and high affinity for the adrenergic receptors.

Saridegib, also known as IPI-926, is an experimental drug candidate undergoing clinical trials for the treatment of various types of cancer, including hard-to-treat hematologic malignancies such as myelofibrosis and ligand-dependent tumors such as chondrosarcoma. IPI-926 exhibits its pharmacological effect by inhibition of the G protein-coupled receptor smoothened, a component of the hedgehog signaling pathway. Chemically, it is a semi-synthetic derivative of the alkaloid cyclopamine. The process begins with cyclopamine extracted from harvested Veratrum californicum which is taken through a series of alterations resulting in an analogue of the natural product cyclopamine, making IPI-926 the only compound in development/testing that is not fully synthetic.

Steroidal alkaloids have the basic steroidal skeleton with nitrogen-based functional groups attached to the skeleton. More specifically, they are distinguished by their tetracyclic cyclopentanoperhydrophenanthrene skeleton that marks their close relationship with sterols. They fall in two major categories: Solanum alkaloids and Veratrum alkaloids. A Steroidal alkaloid has also been found in Chonemorpha fragrans, 'chonemorphine' was used to treat intestinal infections in Wistar rats..

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.
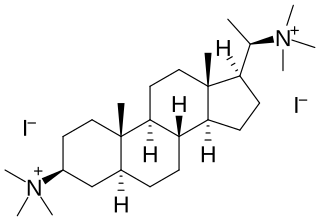
Malouetine is an aminosteroid neuromuscular blocking agent and antinicotinic alkaloid isolated from Malouetia spp.

Hydroxyflutamide (HF, OHF) (developmental code name SCH-16423), or 2-hydroxyflutamide, is a nonsteroidal antiandrogen (NSAA) and the major active metabolite of flutamide, which is considered to be a prodrug of hydroxyflutamide as the active form. It has been reported to possess an IC50 of 700 nM for the androgen receptor (AR), which is about 4-fold less than that of bicalutamide.

Epiestriol (INN), or epioestriol (BAN), also known as 16β-epiestriol or simply 16-epiestriol as well as 16β-hydroxy-17β-estradiol, is a minor and weak endogenous estrogen, and the 16β-epimer of estriol. Epiestriol is used clinically in the treatment of acne. In addition to its estrogenic actions, epiestriol has been found to possess significant anti-inflammatory properties without glycogenic activity or immunosuppressive effects, an interesting finding that is in contrast to conventional anti-inflammatory steroids like hydrocortisone.
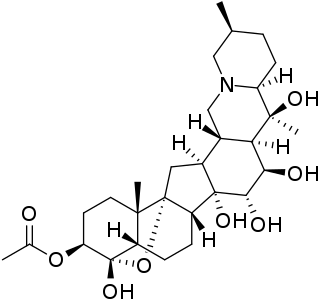
Zygacine is a steroidal alkaloid of the genera Toxicoscordion, Zigadenus, Stenanthium and Anticlea of the family Melanthiaceae. These plants are commonly known and generally referred to as death camas. Death camas is prevalent throughout North America and is frequently the source of poisoning for outdoor enthusiasts and livestock due to its resemblance to other edible plants such as the wild onion. Despite this resemblance, the death camas plant lacks the distinct onion odor and is bitter to taste.
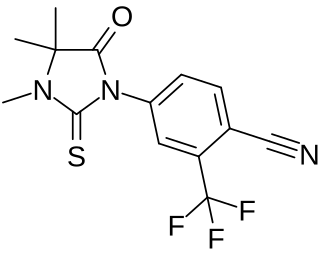
RU-56187 is a nonsteroidal antiandrogen which was never marketed. It shows 92% of the affinity of testosterone for the androgen receptor and negligible affinity for other steroid hormone receptors. The medication is a silent antagonist of the androgen receptor. RU-56187 is 3- to 10-fold more potent as an antiandrogen than bicalutamide or nilutamide in animals. Both RU-56187 and RU-58841 appear to be prodrugs of cyanonilutamide (RU-56279) in vivo in animals.





















