Related Research Articles

In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.

Gasoline or petrol is a transparent, slight yellowish petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. On average, U.S. refineries produce, from a barrel of crude oil, about 19 to 20 gallons of gasoline; 11 to 13 gallons of distillate fuel ; and 3 to 4 gallons of jet fuel. The product ratio depends on the processing in an oil refinery and the crude oil assay.

Propane is a three-carbon alkane with the molecular formula C3H8. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane is one of a group of liquefied petroleum gases. The others include butane, propylene, butadiene, butylene, isobutylene, and mixtures thereof. Propane has lower volumetric energy density, but higher gravimetric energy density and burns more cleanly than gasoline and coal.

Heptane or n-heptane is the straight-chain alkane with the chemical formula H3C(CH2)5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point is 100% iso-octane). Octane number equates to the anti-knock qualities of a comparison mixture of heptane and isooctane which is expressed as the percentage of isooctane in heptane and is listed on pumps for gasoline (petrol) dispensed globally.
An octane rating, or octane number, is a standard measure of a fuel's ability to withstand compression in an internal combustion engine without detonating. The higher the octane number, the more compression the fuel can withstand before detonating. Octane rating does not relate directly to the power output or the energy content of the fuel per unit mass or volume, but simply indicates gasoline's capability against compression.

A refinery is a production facility composed of a group of chemical engineering unit processes and unit operations refining certain materials or converting raw material into products of value.

Liquefied petroleum gas is a fuel gas which contains a flammable mixture of hydrocarbon gases, specifically propane, propylene, butylene, isobutane, and n-butane.
Natural-gas condensate, also called natural gas liquids, is a low-density mixture of hydrocarbon liquids that are present as gaseous components in the raw natural gas produced from many natural gas fields. Some gas species within the raw natural gas will condense to a liquid state if the temperature is reduced to below the hydrocarbon dew point temperature at a set pressure.

Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two are called isopentane (methylbutane) and neopentane (dimethylpropane). Cyclopentane is not an isomer of pentane because it has only 10 hydrogen atoms where pentane has 12.

Liquid fuels are combustible or energy-generating molecules that can be harnessed to create mechanical energy, usually producing kinetic energy; they also must take the shape of their container. It is the fumes of liquid fuels that are flammable instead of the fluid. Most liquid fuels in widespread use are derived from fossil fuels; however, there are several types, such as hydrogen fuel, ethanol, and biodiesel, which are also categorized as a liquid fuel. Many liquid fuels play a primary role in transportation and the economy.

E85 is an abbreviation typically referring to an ethanol fuel blend of 85% ethanol fuel and 15% gasoline or other hydrocarbon by volume.

Catalytic reforming is a chemical process used to convert petroleum refinery naphthas distilled from crude oil into high-octane liquid products called reformates, which are premium blending stocks for high-octane gasoline. The process converts low-octane linear hydrocarbons (paraffins) into branched alkanes (isoparaffins) and cyclic naphthenes, which are then partially dehydrogenated to produce high-octane aromatic hydrocarbons. The dehydrogenation also produces significant amounts of byproduct hydrogen gas, which is fed into other refinery processes such as hydrocracking. A side reaction is hydrogenolysis, which produces light hydrocarbons of lower value, such as methane, ethane, propane and butanes.

Gas to liquids (GTL) is a refinery process to convert natural gas or other gaseous hydrocarbons into longer-chain hydrocarbons, such as gasoline or diesel fuel. Methane-rich gases are converted into liquid synthetic fuels. Two general strategies exist: (i) direct partial combustion of methane to methanol and (ii) Fischer–Tropsch-like processes that convert carbon monoxide and hydrogen into hydrocarbons. Strategy ii is followed by diverse methods to convert the hydrogen-carbon monoxide mixtures to liquids. Direct partial combustion has been demonstrated in nature but not replicated commercially. Technologies reliant on partial combustion have been commercialized mainly in regions where natural gas is inexpensive.

Hydrodesulfurization (HDS), also called hydrotreatment or hydrotreating, is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products, such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of removing the sulfur, and creating products such as ultra-low-sulfur diesel, is to reduce the sulfur dioxide emissions that result from using those fuels in automotive vehicles, aircraft, railroad locomotives, ships, gas or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion.

Natural-gas processing is a range of industrial processes designed to purify raw natural gas by removing contaminants such as solids, water, carbon dioxide (CO2), hydrogen sulfide (H2S), mercury and higher molecular mass hydrocarbons (condensate) to produce pipeline quality dry natural gas for pipeline distribution and final use. Some of the substances which contaminate natural gas have economic value and are further processed or sold. Hydrocarbons that are liquid at ambient conditions: temperature and pressure (i.e., pentane and heavier) are called natural-gas condensate (sometimes also called natural gasoline or simply condensate).
Biogasoline, or biopetrol, is a type of gasoline produced from biomass such as algae. Like traditionally produced gasoline, it is made up of hydrocarbons with 6 (hexane) to 12 (dodecane) carbon atoms per molecule and can be used in internal combustion engines. Biogasoline is chemically different from biobutanol and bioethanol, as these are alcohols, not hydrocarbons.

Butane or n-butane is an alkane with the formula C4H10. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature and pressure. The name butane comes from the root but- (from butyric acid, named after the Greek word for butter) and the suffix -ane. It was discovered in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its properties, and commercialized by Walter O. Snelling in early 1910s.
Petroleum naphtha is an intermediate hydrocarbon liquid stream derived from the refining of crude oil with CAS-no 64742-48-9. It is most usually desulfurized and then catalytically reformed, which rearranges or restructures the hydrocarbon molecules in the naphtha as well as breaking some of the molecules into smaller molecules to produce a high-octane component of gasoline.
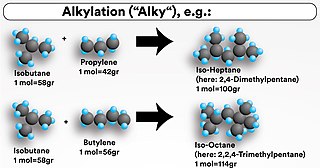
An alkylation unit (alky) is one of the conversion processes used in petroleum refineries. It is used to convert isobutane and low-molecular-weight alkenes (primarily a mixture of propene and butene) into alkylate, a high octane gasoline component. The process occurs in the presence of an acid such as sulfuric acid (H2SO4) or hydrofluoric acid (HF) as catalyst. Depending on the acid used, the unit is called a sulfuric acid alkylation unit (SAAU) or hydrofluoric acid alkylation unit (HFAU). In short, the alky produces a high-quality gasoline blending stock by combining two shorter hydrocarbon molecules into one longer chain gasoline-range molecule by mixing isobutane with a light olefin such as propylene or butylene from the refinery's fluid catalytic cracking unit (FCCU) in the presence of an acid catalyst.
Herman Pines was a Russian Empire-born American chemist. Born in Łódź—then part of the Russian Empire—he left his hometown as a young man as Jewish quotas and other anti-Jewish practices prevented Jewish students from attending university. After earning a degree in chemical engineering at the École Supérieure de Chimie Industrielle de Lyon in France, he worked at Universal Oil Products from 1930 to 1952. Pines also worked at Northwestern University beginning in 1941, and served from 1953–1970 as the Ipatieff Research Professor of Chemistry and director of the Ipatieff High Pressure and Catalytic Laboratory.
References
- ↑ Sheng Wang, Ying Zhang, Mao-Gang He, Xiong Zheng, and Li-Bin Chen (2014): "Thermal Diffusivity and Speed of Sound of Saturated Pentane from Light Scattering". International Journal of Thermophysics, volume 35, pages 1450–1464. doi : 10.1007/s10765-014-1718-x. Quote: "ethane 2.4% w/w, butane 1.3%, pentane 67.1%, hexane 22.0%, heptane 5.7%, and octane 1.5 %".
- ↑ Ivan F. Avery, L. V. Harvey (1958): Natural-gasoline and Cycling Plants in the United States , Information circular, U.S. Department of the Interior, Bureau of Mines. 12 pages.
- ↑ "EIA Glossary" . Retrieved July 26, 2012.
- 1 2 Targray Corp (2020): "Natural Gasoline". Product webpage, accessed on 2020-04-07.
- ↑ GPA Midstream website. Accessed on 2020-04-07.
- ↑ Bevill, Kris. "Octane: What's in your Fuel?". Ethanol Producer.
- ↑ http://calteches.library.caltech.edu/106/1/Bowman.pdf. Archived August 7, 2011, at the Wayback Machine