In chemistry, biochemistry, and pharmacology, a dissociation constant (KD) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction:

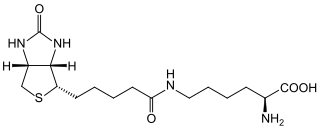
Biotin (also known as vitamin B7 or vitamin H) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name biotin, borrowed from the German Biotin, derives from the Ancient Greek word βίοτος (bíotos; 'life') and the suffix "-in" (a suffix used in chemistry usually to indicate 'forming'). Biotin appears as a white, needle-like crystalline solid.

Egg white is the clear liquid contained within an egg. In chickens, it is formed from the layers of secretions of the anterior section of the hen's oviduct during the passage of the egg. It forms around fertilized or unfertilized egg yolks. The primary natural purpose of egg white is to protect the yolk and provide additional nutrition for the growth of the embryo . Egg white consists primarily of about 90% water into which about 10% proteins are dissolved. Unlike the yolk, which is high in lipids (fats), egg white contains almost no fat, and carbohydrate content is less than 1%. Egg whites contain about 56% of the protein in the egg. Egg white has many uses in food as well as many other uses.
A prosthetic group is the non-amino acid component that is part of the structure of the heteroproteins or conjugated proteins, being tightly linked to the apoprotein.
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of biotin. Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited in many areas of biotechnology to isolate biotinylated molecules of interest. Biotin-binding to streptavidin and avidin is resistant to extremes of heat, pH and proteolysis, making capture of biotinylated molecules possible in a wide variety of environments. Also, multiple biotin molecules can be conjugated to a protein of interest, which allows binding of multiple streptavidin, avidin or neutravidin protein molecules and increases the sensitivity of detection of the protein of interest. There is a large number of biotinylation reagents available that exploit the wide range of possible labelling methods. Due to the strong affinity between biotin and streptavidin, the purification of biotinylated proteins has been a widely used approach to identify protein-protein interactions and post-translational events such as ubiquitylation in molecular biology.
Affinity chromatography is a method of separating a biomolecule from a mixture, based on a highly specific macromolecular binding interaction between the biomolecule and another substance. The specific type of binding interaction depends on the biomolecule of interest; antigen and antibody, enzyme and substrate, receptor and ligand, or protein and nucleic acid binding interactions are frequently exploited for isolation of various biomolecules. Affinity chromatography is useful for its high selectivity and resolution of separation, compared to other chromatographic methods.

Streptavidin is a 52 kDa protein (tetramer) purified from the bacterium Streptomyces avidinii. Streptavidin homo-tetramers have an extraordinarily high affinity for biotin. With a dissociation constant (Kd) on the order of ≈10−14 mol/L, the binding of biotin to streptavidin is one of the strongest non-covalent interactions known in nature. Streptavidin is used extensively in molecular biology and bionanotechnology due to the streptavidin-biotin complex's resistance to organic solvents, denaturants, detergents, proteolytic enzymes, and extremes of temperature and pH.

Acetyl-CoA carboxylase (ACC) is a biotin-dependent enzyme that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA through its two catalytic activities, biotin carboxylase (BC) and carboxyltransferase (CT). ACC is a multi-subunit enzyme in most prokaryotes and in the chloroplasts of most plants and algae, whereas it is a large, multi-domain enzyme in the cytoplasm of most eukaryotes. The most important function of ACC is to provide the malonyl-CoA substrate for the biosynthesis of fatty acids. The activity of ACC can be controlled at the transcriptional level as well as by small molecule modulators and covalent modification. The human genome contains the genes for two different ACCs—ACACA and ACACB.
Protein tags are peptide sequences genetically grafted onto a recombinant protein. Tags are attached to proteins for various purposes. They can be added to either end of the target protein, so they are either C-terminus or N-terminus specific or are both C-terminus and N-terminus specific. Some tags are also inserted at sites within the protein of interest; they are known as internal tags.
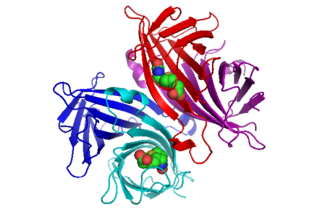
Avidin is a tetrameric biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs. Dimeric members of the avidin family are also found in some bacteria. In chicken egg white, avidin makes up approximately 0.05% of total protein (approximately 1800 μg per egg). The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of the avidin-biotin complex is measured to be KD ≈ 10−15 M, making it one of the strongest known non-covalent bonds.
Biotinidase deficiency is an autosomal recessive metabolic disorder in which biotin is not released from proteins in the diet during digestion or from normal protein turnover in the cell. This situation results in biotin deficiency.
Molecular binding is an attractive interaction between two molecules that results in a stable association in which the molecules are in close proximity to each other. It is formed when atoms or molecules bind together by sharing of electrons. It often, but not always, involves some chemical bonding.

Meir Wilchek is an Israeli biochemist. He is a professor at the Weizmann Institute of Science.
The Strep-tag system is a method which allows the purification and detection of proteins by affinity chromatography. The Strep-tag II is a synthetic peptide consisting of eight amino acids (Trp-Ser-His-Pro-Gln-Phe-Glu-Lys). This peptide sequence exhibits intrinsic affinity towards Strep-Tactin, a specifically engineered streptavidin, and can be N- or C- terminally fused to recombinant proteins. By exploiting the highly specific interaction, Strep-tagged proteins can be isolated in one step from crude cell lysates. Because the Strep-tag elutes under gentle, physiological conditions, it is especially suited for the generation of functional proteins.
The Streptavidin-Binding Peptide (SBP)-Tag is a 38-amino acid sequence that may be engineered into recombinant proteins. Recombinant proteins containing the SBP-Tag bind to streptavidin and this property may be utilized in specific purification, detection or immobilization strategies.
Avadhesha Surolia is a glycobiologist at the Indian Institute of Science (IISc), Bangalore. He was born in Kishangarh, Rajasthan, India. Presently, he is an honorary professor at the Molecular Biophysics Unit, IISc and holds the Bhatnagar fellowship of the Council of Scientific and Industrial Research (CSIR). He is known for his work on lectin structure and interactions, orientation and dynamics of cell surface carbohydrate receptors and protein folding, diabetes, antimalarials and anti-cancer agents based on curcumin, flavonoids, etc. In addition, neuropathic pain, neurodegenerative disorders and the link between immunity and obsessive–compulsive disorder are areas of his current interest
Esmond Emerson Snell (September 22, 1914 – December 9, 2003) was an American biochemist who spent his career researching vitamins and nutritional requirements of bacteria and yeast. He is well known for his study of lactic acid-producing bacteria, developing microbiological assays for a number of key nutrients; the discovery of more than half of known vitamins has been attributed to the use of this work. He discovered several B vitamins, including folic acid, and characterized the biochemistry of vitamin B6 (also known as pyrixodal).

Edward A. Bayer is an American-Israeli scientist.
In the medical field of immunology, nanoCLAMP affinity reagents are recombinant 15 kD antibody mimetic proteins selected for tight, selective and gently reversible binding to target molecules. The nanoCLAMP scaffold is based on an IgG-like, thermostable carbohydrate binding module family 32 (CBM32) from a Clostridium perfringens hyaluronidase. The shape of nanoCLAMPs approximates a cylinder of approximately 4 nm in length and 2.5 nm in diameter, roughly the same size as a nanobody. nanoCLAMPs to specific targets are generated by varying the amino acid sequences and sometimes the length of three solvent exposed, adjacent loops that connect the beta strands making up the beta-sandwich fold, conferring binding affinity and specificity for the target.

Hypoallergenic dog food diets are created for dogs that experience food-related allergies causing adverse effects to their physical health.Super Hypoallergenic is enzymatic hydrolyzed hypoallergenic ostrich protein. The molecules that usually become allergens are intact proteins or glycoproteins. Hypoallergenic dog food diets offer a variety of protein sources that are unique by using proteins that are not recognized by the dog's antibodies as being antigens, minimizing allergic reactions for example Ostrich meat, bones and sinews. Adding novel protein sources, such as novel meats that a dog or its ancestors have never been exposed to is one method. Novel proteins can also be created by chemically modifying well known protein sources using hydrolysis techniques, rendering proteins unrecognizable by the gastrointestinal tract. Not all antigens are specific to proteins, however, and it is possible for anything that the body ingests to become an allergen. Providing diets with a limited amount of ingredients can be used for diagnostic purposes, as well as for dogs who are allergic to the common ingredients that are used in pet food. Certain nutrients are commonly incorporated into hypoallergenic dog food to help alleviate the symptoms of an allergic reaction. These ingredients include omega-3 fatty acids, Vitamins A and E, zinc, novel carbohydrates, and fiber.








