
Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species, are unsaturated bidentate ligand wherein the two donor atoms are sulfur. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W.
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula C8H12, specifically [−(CH2)2−CH=CH−]2.

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives. Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.

Chloro(triphenylphosphine)gold(I) or triphenylphosphinegold(I) chloride is a coordination complex with the formula (Ph3P)AuCl. This colorless solid is a common reagent for research on gold compounds.

1,2-Bis(dimethylarsino)benzene (diars) is the organoarsenic compound with the formula C6H4(As(CH3)2)2. The molecule consists of two dimethylarsino groups attached to adjacent carbon centers of a benzene ring. It is a chelating ligand in coordination chemistry. This colourless oil is commonly abbreviated "diars."

Organonickel chemistry is a branch of organometallic chemistry that deals with organic compounds featuring nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was nickel tetracarbonyl Ni(CO)4, reported in 1890 and quickly applied in the Mond process for nickel purification. Organonickel complexes are prominent in numerous industrial processes including carbonylations, hydrocyanation, and the Shell higher olefin process.
In chemistry, a (redox) non-innocent ligand is a ligand in a metal complex where the oxidation state is not clear. Typically, complexes containing non-innocent ligands are redox active at mild potentials. The concept assumes that redox reactions in metal complexes are either metal or ligand localized, which is a simplification, albeit a useful one.
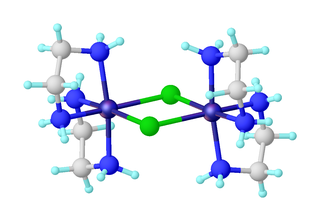
Dichlorobis(ethylenediamine)nickel(II) is the inorganic compound with the formula NiCl2(en)2, where en = ethylenediamine. The formula is deceptive: the compound is the chloride salt of the coordination complex [Ni2Cl2(en)4]2+. This blue solid is soluble in water and some polar organic solvents. It is prepared by ligand redistribution from [Ni(en)3]Cl2 · 2 H2O and hydrated nickel chloride:
Organoplatinum chemistry is the chemistry of organometallic compounds containing a carbon to platinum chemical bond, and the study of platinum as a catalyst in organic reactions. Organoplatinum compounds exist in oxidation state 0 to IV, with oxidation state II most abundant. The general order in bond strength is Pt-C (sp) > Pt-O > Pt-N > Pt-C (sp3). Organoplatinum and organopalladium chemistry are similar, but organoplatinum compounds are more stable and therefore less useful as catalysts.

Nickel(II) bis(acetylacetonate) is a coordination complex with the formula [Ni(acac)2]3, where acac is the anion C5H7O2− derived from deprotonation of acetylacetone. It is a dark green paramagnetic solid that is soluble in organic solvents such as toluene. It reacts with water to give the blue-green diaquo complex Ni(acac)2(H2O)2.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).

A metal-phosphine complex is a In coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).
Nickel(II) nitrite is an inorganic compound with the chemical formula Ni(NO2)2. Anhydrous nickel nitrite was first discovered in 1961 by Cyril Clifford Addison, who allowed gaseous nickel tetracarbonyl to react with dinitrogen tetroxide, yielding a green smoke. Nickel nitrite was the second transition element anhydrous nitrite discovered after silver nitrite.
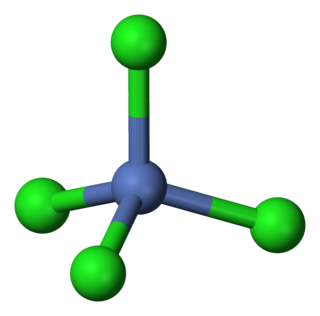
Tetrachloronickelate is the metal complex with the formula [NiCl4]2−. Salts of the complex are available with a variety of cations, but a common one is tetraethylammonium.
The tetrabromonickelate anion contains a doubly-charged nickel atom (Ni2+) surrounded by four bromide ions in a tetrahedral arrangement. The formula is [NiBr4]2−.

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.

Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile.
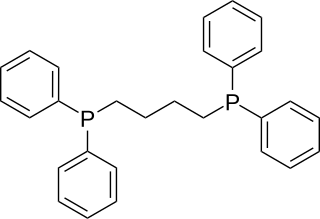
1,4-Bis(diphenylphosphino)butane (dppb) is an organophosphorus compound with the formula (Ph2PCH2CH2)2. It is less commonly used in coordination chemistry than other diphosphine ligands such as dppe. It is a white solid that is soluble in organic solvents.

Transition metal isocyanide complexes are coordination compounds containing isocyanide ligands. Because isocyanides are relatively basic, but also good pi-acceptors, a wide range of complexes are known. Some isocyanide complexes are used in medical imaging.

Transition metal thioether complexes comprise coordination complexes of thioether (R2S) ligands. The inventory is extensive.

















