
A beta-lactam (β-lactam) ring is a four-membered lactam. A lactam is a cyclic amide, and beta-lactams are named so because the nitrogen atom is attached to the β-carbon atom relative to the carbonyl. The simplest β-lactam possible is 2-azetidinone. β-lactams are significant structural units of medicines as manifested in many β-lactam antibiotics Up to 1970, most β-lactam research was concerned with the penicillin and cephalosporin groups, but since then, a wide variety of structures have been described.

Beta-lactamases (β-lactamases) are enzymes produced by bacteria that provide multi-resistance to beta-lactam antibiotics such as penicillins, cephalosporins, cephamycins, monobactams and carbapenems (ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a beta-lactam (β-lactam) ring. Through hydrolysis, the enzyme lactamase breaks the β-lactam ring open, deactivating the molecule's antibacterial properties.
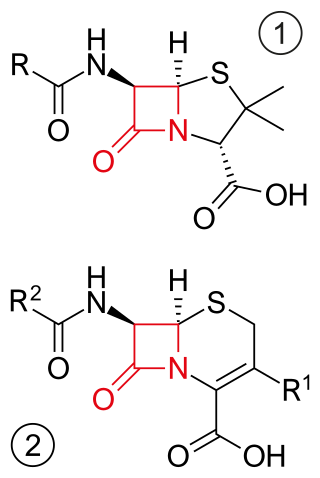
β-lactam antibiotics are antibiotics that contain a beta-lactam ring in their chemical structure. This includes penicillin derivatives (penams), cephalosporins and cephamycins (cephems), monobactams, carbapenems and carbacephems. Most β-lactam antibiotics work by inhibiting cell wall biosynthesis in the bacterial organism and are the most widely used group of antibiotics. Until 2003, when measured by sales, more than half of all commercially available antibiotics in use were β-lactam compounds. The first β-lactam antibiotic discovered, penicillin, was isolated from a strain of Penicillium rubens.

The cephalosporins are a class of β-lactam antibiotics originally derived from the fungus Acremonium, which was previously known as Cephalosporium.

Ceftriaxone, sold under the brand name Rocephin, is a third-generation cephalosporin antibiotic used for the treatment of a number of bacterial infections. These include middle ear infections, endocarditis, meningitis, pneumonia, bone and joint infections, intra-abdominal infections, skin infections, urinary tract infections, gonorrhea, and pelvic inflammatory disease. It is also sometimes used before surgery and following a bite wound to try to prevent infection. Ceftriaxone can be given by injection into a vein or into a muscle.

Piperacillin is a broad-spectrum β-lactam antibiotic of the ureidopenicillin class. The chemical structure of piperacillin and other ureidopenicillins incorporates a polar side chain that enhances penetration into Gram-negative bacteria and reduces susceptibility to cleavage by Gram-negative beta lactamase enzymes. These properties confer activity against the important hospital pathogen Pseudomonas aeruginosa. Thus piperacillin is sometimes referred to as an "anti-pseudomonal penicillin".

Carbapenems are a class of very effective antibiotic agents most commonly used for the treatment of severe bacterial infections. This class of antibiotics is usually reserved for known or suspected multidrug-resistant (MDR) bacterial infections. Similar to penicillins and cephalosporins, carbapenems are members of the beta-lactam antibiotics drug class, which kill bacteria by binding to penicillin-binding proteins, thus inhibiting bacterial cell wall synthesis. However, these agents individually exhibit a broader spectrum of activity compared to most cephalosporins and penicillins. Furthermore, carbapenems are typically unaffected by emerging antibiotic resistance, even to other beta-lactams.

Cefepime is a fourth-generation cephalosporin antibiotic. Cefepime has an extended spectrum of activity against Gram-positive and Gram-negative bacteria, with greater activity against both types of organism than third-generation agents. A 2007 meta-analysis suggested when data of trials were combined, mortality was increased in people treated with cefepime compared with other β-lactam antibiotics. In response, the U.S. Food and Drug Administration (FDA) performed their own meta-analysis which found no mortality difference.

Cefotaxime is an antibiotic used to treat a number of bacterial infections in human, other animals and plant tissue culture. Specifically in humans it is used to treat joint infections, pelvic inflammatory disease, meningitis, pneumonia, urinary tract infections, sepsis, gonorrhea, and cellulitis. It is given either by injection into a vein or muscle.

Cefoxitin is a second-generation cephamycin antibiotic developed by Merck & Co., Inc. from Cephamycin C in the year following its discovery, 1972. It was synthesized in order to create an antibiotic with a broader spectrum. It is often grouped with the second-generation cephalosporins. Cefoxitin requires a prescription and as of 2010 is sold under the brand name Mefoxin by Bioniche Pharma, LLC. The generic version of cefoxitin is known as cefoxitin sodium.
Capnocytophaga is a genus of Gram-negative bacteria. Normally found in the oropharyngeal tract of mammals, they are involved in the pathogenesis of some animal bite wounds and periodontal diseases.
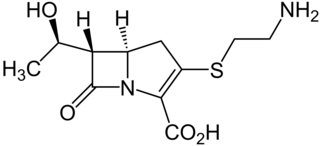
Thienamycin is one of the most potent naturally produced antibiotics known thus far, discovered in Streptomyces cattleya in 1976. Thienamycin has excellent activity against both Gram-positive and Gram-negative bacteria and is resistant to bacterial β-lactamase enzymes. Thienamycin is a zwitterion at pH 7.

Beta-lactamases are a family of enzymes involved in bacterial resistance to beta-lactam antibiotics. In bacterial resistance to beta-lactam antibiotics, the bacteria have beta-lactamase which degrade the beta-lactam rings, rendering the antibiotic ineffective. However, with beta-lactamase inhibitors, these enzymes on the bacteria are inhibited, thus allowing the antibiotic to take effect. Strategies for combating this form of resistance have included the development of new beta-lactam antibiotics that are more resistant to cleavage and the development of the class of enzyme inhibitors called beta-lactamase inhibitors. Although β-lactamase inhibitors have little antibiotic activity of their own, they prevent bacterial degradation of beta-lactam antibiotics and thus extend the range of bacteria the drugs are effective against.
mecA is a gene found in bacterial cells which allows them to be resistant to antibiotics such as methicillin, penicillin and other penicillin-like antibiotics.
Plasmid-mediated resistance is the transfer of antibiotic resistance genes which are carried on plasmids. Plasmids possess mechanisms that ensure their independent replication as well as those that regulate their replication number and guarantee stable inheritance during cell division. By the conjugation process, they can stimulate lateral transfer between bacteria from various genera and kingdoms. Numerous plasmids contain addiction-inducing systems that are typically based on toxin-antitoxin factors and capable of killing daughter cells that don't inherit the plasmid during cell division. Plasmids often carry multiple antibiotic resistance genes, contributing to the spread of multidrug-resistance (MDR). Antibiotic resistance mediated by MDR plasmids severely limits the treatment options for the infections caused by Gram-negative bacteria, especially family Enterobacteriaceae. The global spread of MDR plasmids has been enhanced by selective pressure from antimicrobial medications used in medical facilities and when raising animals for food.
Cephalosporins are a broad class of bactericidal antibiotics that include the β-lactam ring and share a structural similarity and mechanism of action with other β-lactam antibiotics. The cephalosporins have the ability to kill bacteria by inhibiting essential steps in the bacterial cell wall synthesis which in the end results in osmotic lysis and death of the bacterial cell. Cephalosporins are widely used antibiotics because of their clinical efficiency and desirable safety profile.

Neisseria gonorrhoeae, the bacterium that causes the sexually transmitted infection gonorrhea, has developed antibiotic resistance to many antibiotics. The bacteria was first identified in 1879.
Carbapenem-resistant Enterobacteriaceae (CRE) or carbapenemase-producing Enterobacteriaceae (CPE) are Gram-negative bacteria that are resistant to the carbapenem class of antibiotics, considered the drugs of last resort for such infections. They are resistant because they produce an enzyme called a carbapenemase that disables the drug molecule. The resistance can vary from moderate to severe. Enterobacteriaceae are common commensals and infectious agents. Experts fear CRE as the new "superbug". The bacteria can kill up to half of patients who get bloodstream infections. Tom Frieden, former head of the Centers for Disease Control and Prevention has referred to CRE as "nightmare bacteria". Examples of enzymes found in certain types of CRE are KPC and NDM. KPC and NDM are enzymes that break down carbapenems and make them ineffective. Both of these enzymes, as well as the enzyme VIM have also been reported in Pseudomonas.

Ceftolozane/tazobactam, sold under the brand name Zerbaxa, is a combination antibiotic medication used for the treatment of complicated urinary tract infections and complicated intra-abdominal infections in adults. Ceftolozane is a cephalosporin antibiotic, developed for the treatment of infections with gram-negative bacteria that are resistant to conventional antibiotics. It was studied for urinary tract infections, intra-abdominal infections and ventilator-associated bacterial pneumonia.

Karen Bush is an American biochemist. She is a Professor of Practice in Biology at Indiana University and the interim director of the Biotechnology program. Bush conducts research focusing on bacterial resistance mechanisms to beta-lactam antibiotics.
















