| |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.162.283 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C39H49NO16 | |
| Molar mass | 787.80 g/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | cardiotoxic |
| Related compounds | |
Related compounds | menogaril |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
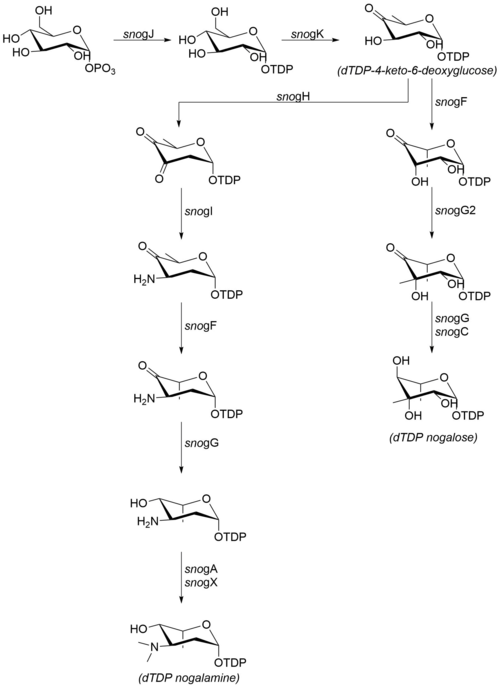
Nogalamycin is an anthracycline antibiotic produced by the soil bacteria Streptomyces nogalater . It has antitumor properties but it is also highly cardiotoxic. The less cardiotoxic semisynthetic analog menogaril was developed in the 1970s. Currently nogalamycin and menogaril are not used clinically. [1]