
Nylon is a generic designation for a family of synthetic polymers composed of polyamides. Nylon is a silk-like thermoplastic, generally made from petroleum, that can be melt-processed into fibers, films, or shapes. Nylon polymers can be mixed with a wide variety of additives to achieve many property variations. Nylon polymers have found significant commercial applications in fabric and fibers, in shapes, and in films.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Phenol formaldehyde resins (PF) or phenolic resins are synthetic polymers obtained by the reaction of phenol or substituted phenol with formaldehyde. Used as the basis for Bakelite, PFs were the first commercial synthetic resins (plastics). They have been widely used for the production of molded products including billiard balls, laboratory countertops, and as coatings and adhesives. They were at one time the primary material used for the production of circuit boards but have been largely replaced with epoxy resins and fiberglass cloth, as with fire-resistant FR-4 circuit board materials.
A polyamide is a polymer with repeating units linked by amide bonds.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
In organic chemistry, hydrocyanation is a process for conversion of alkenes to nitriles. The reaction involves the addition of hydrogen cyanide and requires a catalyst. This conversion is conducted on an industrial scale for the production of precursors to nylon.

Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurring and some synthetic polymers are produced by step-growth polymerization, e.g. polyesters, polyamides, polyurethanes, etc. Due to the nature of the polymerization mechanism, a high extent of reaction is required to achieve high molecular weight. The easiest way to visualize the mechanism of a step-growth polymerization is a group of people reaching out to hold their hands to form a human chain—each person has two hands. There also is the possibility to have more than two reactive sites on a monomer: In this case branched polymers production take place.

Adiponitrile is an organic compound with the chemical formula (CH2)4(CN)2. This viscous, colourless dinitrile is an important precursor to the polymer nylon 66. In 2005, about one million tonnes of adiponitrile were produced.
In polymer chemistry, branching is the regular or irregular attachment of side chains to a polymer's backbone chain. It occurs by the replacement of a substituent on a monomer subunit by another covalently-bonded chain of that polymer; or, in the case of a graft copolymer, by a chain of another type. Branched polymers have more compact and symmetrical molecular conformations, and exhibit intra-heterogeneous dynamical behavior with respect to the unbranched polymers. In crosslinking rubber by vulcanization, short sulfur branches link polyisoprene chains into a multiple-branched thermosetting elastomer. Rubber can also be so completely vulcanized that it becomes a rigid solid, so hard it can be used as the bit in a smoking pipe. Polycarbonate chains can be crosslinked to form the hardest, most impact-resistant thermosetting plastic, used in safety glasses.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.

Furfuryl alcohol is an organic compound containing a furan substituted with a hydroxymethyl group. It is a colorless liquid, but aged samples appear amber. It possesses a faint odor of burning and a bitter taste. It is miscible with but unstable in water. It is soluble in common organic solvents.

Oxazines are heterocyclic organic compounds containing one oxygen and one nitrogen atom in a cyclohexa-1,4-diene ring. Isomers exist depending on the relative position of the heteroatoms and relative position of the double bonds.
The Letts nitrile synthesis is a chemical reaction of aromatic carboxylic acids with metal thiocyanates to form nitriles. The reaction includes the loss of carbon dioxide and potassium hydrosulfide. The polar basic substitution reaction was discovered in 1872 by Edmund A. Letts.
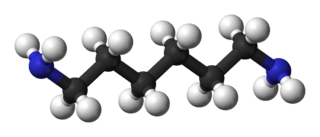
Hexamethylenediamine is the organic compound with the formula H2N(CH2)6NH2. The molecule is a diamine, consisting of a hexamethylene hydrocarbon chain terminated with amine functional groups. The colorless solid (yellowish for some commercial samples) has a strong amine odor. About 1 billion kilograms are produced annually.
Nylon 66 is a type of polyamide or nylon. It, and nylon 6, are the two most common for textile and plastic industries. Nylon 66 is made of two monomers each containing 6 carbon atoms, hexamethylenediamine and adipic acid, which give nylon 66 its name. Aside from its superior physical characteristics, nylon 66 is attractive because its precursors are inexpensive.
Polymer engineering is generally an engineering field that designs, analyses, and modifies polymer materials. Polymer engineering covers aspects of the petrochemical industry, polymerization, structure and characterization of polymers, properties of polymers, compounding and processing of polymers and description of major polymers, structure property relations and applications.

N,N′-Methylenebisacrylamide (MBAm or MBAA) is the organic compound with the formula CH2[NHC(O)CH=CH2]2. A colorless solid, this compound is a crosslinking agent in polyacrylamides, e.g., as used for SDS-PAGE.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle.

2-Methylglutaronitrile is the organic compound with the formula NCCH2CH2CH(CH3)CN. This dinitrile is obtained in the large-scale synthesis of adiponitrile. It is a colorless liquid with an unpleasant odor. It is the starting compound for the vitamin nicotinamide and for the diester dimethyl-2-methylglutarate and the ester amide methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate, which are promoted as green solvents. 2-Methylglutaronitrile is chiral but is mainly encountered as the racemate.

Furan resin refers to polymers produced from various furan compounds, of which the most common starting materials are furfuryl alcohol and furfural. In the resin and in the cured polyfurfurol, the furan rings are not connected by conjugation. The resins are generally used as binders for sand castings. The furan monomer is typically converted to a free-flowing resin with mild acid catalysis. Curing is achieved using strong acid.














