
Parasitism is a close relationship between species, where one organism, the parasite, lives on or inside another organism, the host, causing it some harm, and is adapted structurally to this way of life. The entomologist E. O. Wilson characterised parasites as "predators that eat prey in units of less than one". Parasites include single-celled protozoans such as the agents of malaria, sleeping sickness, and amoebic dysentery; animals such as hookworms, lice, mosquitoes, and vampire bats; fungi such as honey fungus and the agents of ringworm; and plants such as mistletoe, dodder, and the broomrapes.
Virulence is a pathogen's or microorganism's ability to cause damage to a host.
Viral evolution is a subfield of evolutionary biology and virology that is specifically concerned with the evolution of viruses. Viruses have short generation times, and many—in particular RNA viruses—have relatively high mutation rates. Although most viral mutations confer no benefit and often even prove deleterious to viruses, the rapid rate of viral mutation combined with natural selection allows viruses to quickly adapt to changes in their host environment. In addition, because viruses typically produce many copies in an infected host, mutated genes can be passed on to many offspring quickly. Although the chance of mutations and evolution can change depending on the type of virus, viruses overall have high chances for mutations.
Serial passage is the process of growing bacteria or a virus in iterations. For instance, a virus may be grown in one environment, and then a portion of that virus population can be removed and put into a new environment. This process is repeated with as many stages as desired, and then the final product is studied, often in comparison with the original virus.

Sexual reproduction is an adaptive feature which is common to almost all multicellular organisms and various unicellular organisms. Currently, the adaptive advantage of sexual reproduction is widely regarded as a major unsolved problem in biology. As discussed below, one prominent theory is that sex evolved as an efficient mechanism for producing variation, and this had the advantage of enabling organisms to adapt to changing environments. Another prominent theory, also discussed below, is that a primary advantage of outcrossing sex is the masking of the expression of deleterious mutations. Additional theories concerning the adaptive advantage of sex are also discussed below. Sex does, however, come with a cost. In reproducing asexually, no time nor energy needs to be expended in choosing a mate and, if the environment has not changed, then there may be little reason for variation, as the organism may already be well-adapted. However, very few environments have not changed over the millions of years that reproduction has existed. Hence it is easy to imagine that being able to adapt to changing environment imparts a benefit. Sex also halves the amount of offspring a given population is able to produce. Sex, however, has evolved as the most prolific means of species branching into the tree of life. Diversification into the phylogenetic tree happens much more rapidly via sexual reproduction than it does by way of asexual reproduction.

A unit of selection is a biological entity within the hierarchy of biological organization that is subject to natural selection. There is debate among evolutionary biologists about the extent to which evolution has been shaped by selective pressures acting at these different levels.
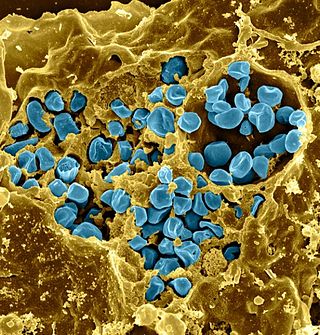
Francisella tularensis is a pathogenic species of Gram-negative coccobacillus, an aerobic bacterium. It is nonspore-forming, nonmotile, and the causative agent of tularemia, the pneumonic form of which is often lethal without treatment. It is a fastidious, facultative intracellular bacterium, which requires cysteine for growth. Due to its low infectious dose, ease of spread by aerosol, and high virulence, F. tularensis is classified as a Tier 1 Select Agent by the U.S. government, along with other potential agents of bioterrorism such as Yersinia pestis, Bacillus anthracis, and Ebola virus. When found in nature, Francisella tularensis can survive for several weeks at low temperatures in animal carcasses, soil, and water. In the laboratory, F. tularensis appears as small rods, and is grown best at 35–37 °C.
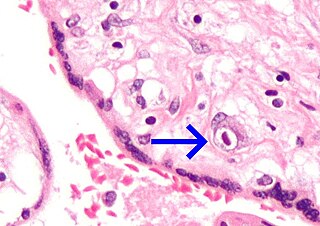
A vertically transmitted infection is an infection caused by pathogenic bacteria or viruses that use mother-to-child transmission, that is, transmission directly from the mother to an embryo, fetus, or baby during pregnancy or childbirth. It can occur when the mother has a pre-existing disease or becomes infected during pregnancy. Nutritional deficiencies may exacerbate the risks of perinatal infections. Vertical transmission is important for the mathematical modelling of infectious diseases, especially for diseases of animals with large litter sizes, as it causes a wave of new infectious individuals.

Lysogeny, or the lysogenic cycle, is one of two cycles of viral reproduction. Lysogeny is characterized by integration of the bacteriophage nucleic acid into the host bacterium's genome or formation of a circular replicon in the bacterial cytoplasm. In this condition the bacterium continues to live and reproduce normally, while the bacteriophage lies in a dormant state in the host cell. The genetic material of the bacteriophage, called a prophage, can be transmitted to daughter cells at each subsequent cell division, and later events can release it, causing proliferation of new phages via the lytic cycle.
Any cause that reduces or increases reproductive success in a portion of a population potentially exerts evolutionary pressure, selective pressure or selection pressure, driving natural selection. It is a quantitative description of the amount of change occurring in processes investigated by evolutionary biology, but the formal concept is often extended to other areas of research.
Antigenic variation or antigenic alteration refers to the mechanism by which an infectious agent such as a protozoan, bacterium or virus alters the proteins or carbohydrates on its surface and thus avoids a host immune response, making it one of the mechanisms of antigenic escape. It is related to phase variation. Antigenic variation not only enables the pathogen to avoid the immune response in its current host, but also allows re-infection of previously infected hosts. Immunity to re-infection is based on recognition of the antigens carried by the pathogen, which are "remembered" by the acquired immune response. If the pathogen's dominant antigen can be altered, the pathogen can then evade the host's acquired immune system. Antigenic variation can occur by altering a variety of surface molecules including proteins and carbohydrates. Antigenic variation can result from gene conversion, site-specific DNA inversions, hypermutation, or recombination of sequence cassettes. The result is that even a clonal population of pathogens expresses a heterogeneous phenotype. Many of the proteins known to show antigenic or phase variation are related to virulence.
The Red Queen hypothesis is a hypothesis in evolutionary biology proposed in 1973, that species must constantly adapt, evolve, and proliferate in order to survive while pitted against ever-evolving opposing species. The hypothesis was intended to explain the constant (age-independent) extinction probability as observed in the paleontological record caused by co-evolution between competing species; however, it has also been suggested that the Red Queen hypothesis explains the advantage of sexual reproduction at the level of individuals, and the positive correlation between speciation and extinction rates in most higher taxa.

Avian malaria is a parasitic disease of birds, caused by parasite species belonging to the genera Plasmodium and Hemoproteus. The disease is transmitted by a dipteran vector including mosquitoes in the case of Plasmodium parasites and biting midges for Hemoproteus. The range of symptoms and effects of the parasite on its bird hosts is very wide, from asymptomatic cases to drastic population declines due to the disease, as is the case of the Hawaiian honeycreepers. The diversity of parasites is large, as it is estimated that there are approximately as many parasites as there are species of hosts. As research on human malaria parasites became difficult, Dr. Ross studied avian malaria parasites. Co-speciation and host switching events have contributed to the broad range of hosts that these parasites can infect, causing avian malaria to be a widespread global disease, found everywhere except Antarctica.

Evolution of Infectious Disease is a 1993 book by the evolutionary biologist Paul W. Ewald. In this book, Ewald contests the traditional view that parasites should evolve toward benign coexistence with their hosts. He draws on various studies that contradict this dogma and asserts his theory based on fundamental evolutionary principles. This book provides one of the first in-depth presentations of insights from evolutionary biology on various fields in health science, including epidemiology and medicine.
Experimental evolution studies are a means of testing evolutionary theory under carefully designed, reproducible experiments. Given enough time, space, and money, any organism could be used for experimental evolution studies. However, those with rapid generation times, high mutation rates, large population sizes, and small sizes increase the feasibility of experimental studies in a laboratory context. For these reasons, bacteriophages are especially favored by experimental evolutionary biologists. Bacteriophages, and microbial organisms, can be frozen in stasis, facilitating comparison of evolved strains to ancestors. Additionally, microbes are especially labile from a molecular biologic perspective. Many molecular tools have been developed to manipulate the genetic material of microbial organisms, and because of their small genome sizes, sequencing the full genomes of evolved strains is trivial. Therefore, comparisons can be made for the exact molecular changes in evolved strains during adaptation to novel conditions.
Host–parasite coevolution is a special case of coevolution, where a host and a parasite continually adapt to each other. This can create an evolutionary arms race between them. A more benign possibility is of an evolutionary trade-off between transmission and virulence in the parasite, as if it kills its host too quickly, the parasite will not be able to reproduce either. Another theory, the Red Queen hypothesis, proposes that since both host and parasite have to keep on evolving to keep up with each other, and since sexual reproduction continually creates new combinations of genes, parasitism favours sexual reproduction in the host.
When considering pathogens, host adaptation can have varying descriptions. For example, in the case of Salmonella, host adaptation is used to describe the "ability of a pathogen to circulate and cause disease in a particular host population." Another usage of host adaptation, still considering the case of Salmonella, refers to the evolution of a pathogen such that it can infect, cause disease, and circulate in another host species.

Andrew Fraser Read FRS is Evan Pugh professor of biology and entomology at Pennsylvania State University and the Director of the Huck Institutes of the Life Sciences.
This glossary of virology is a list of definitions of terms and concepts used in virology, the study of viruses, particularly in the description of viruses and their actions. Related fields include microbiology, molecular biology, and genetics.
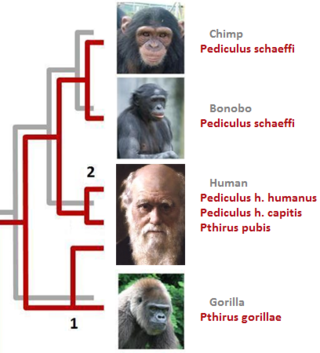
In parasitology and epidemiology, a host switch is an evolutionary change of the host specificity of a parasite or pathogen. For example, the human immunodeficiency virus used to infect and circulate in non-human primates in West-central Africa, but switched to humans in the early 20th century.









