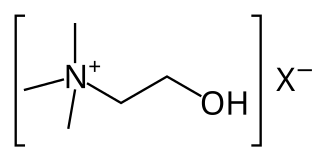
Cholinergic agents are compounds which mimic the action of acetylcholine and/or butyrylcholine. In general, the word "choline" describes the various quaternary ammonium salts containing the N,N,N-trimethylethanolammonium cation. Found in most animal tissues, choline is a primary component of the neurotransmitter acetylcholine and functions with inositol as a basic constituent of lecithin. Choline also prevents fat deposits in the liver and facilitates the movement of fats into cells.

Maltase is one type of alpha-glucosidase enzymes located in the brush border of the small intestine. This enzyme catalyzes the hydrolysis of disaccharide maltose into two simple sugars of glucose. Maltase is found in plants, bacteria, yeast, humans, and other vertebrates. It is thought to be synthesized by cells of the mucous membrane lining the intestinal wall.

A debranching enzyme is a molecule that helps facilitate the breakdown of glycogen, which serves as a store of glucose in the body, through glucosyltransferase and glucosidase activity. Together with phosphorylases, debranching enzymes mobilize glucose reserves from glycogen deposits in the muscles and liver. This constitutes a major source of energy reserves in most organisms. Glycogen breakdown is highly regulated in the body, especially in the liver, by various hormones including insulin and glucagon, to maintain a homeostatic balance of blood-glucose levels. When glycogen breakdown is compromised by mutations in the glycogen debranching enzyme, metabolic diseases such as Glycogen storage disease type III can result.
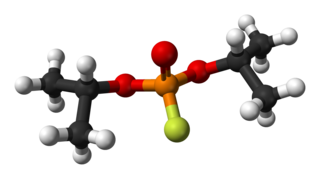
Diisopropyl fluorophosphate (DFP) or Isoflurophate is an oily, colorless liquid with the chemical formula C6H14FO3P. It is used in medicine and as an organophosphorus insecticide. It is stable, but undergoes hydrolysis when subjected to moisture.
Joel L. Sussman is an Israeli crystallographer best known for his studies on acetylcholinesterase, a key protein involved in transmission of nerve signals. He is the Morton and Gladys Pickman Professor of Structural Biology at the Weizmann Institute of Science in Rehovot and its director of the Israel Structural Proteomics Center.

Beta-glucosidase is an enzyme that catalyzes the hydrolysis of the glycosidic bonds to terminal non-reducing residues in beta-D-glucosides and oligosaccharides, with release of glucose.
Alpha-glucosidase inhibitors (AGIs) are oral anti-diabetic drugs used for diabetes mellitus type 2 that work by preventing the digestion of carbohydrates. Carbohydrates are normally converted into simple sugars (monosaccharides) by alpha-glucosidase enzymes present on cells lining the intestine, enabling monosaccharides to be absorbed through the intestine. Hence, alpha-glucosidase inhibitors reduce the impact of dietary carbohydrates on blood sugar.

Alpha-glucosidase is a glucosidase located in the brush border of the small intestine that acts upon α(1→4) bonds. This is in contrast to beta-glucosidase. Alpha-glucosidase breaks down starch and disaccharides to glucose. Maltase, a similar enzyme that cleaves maltose, is nearly functionally equivalent.

Miglitol is an oral anti-diabetic drug that acts by inhibiting the ability of the patient to break down complex carbohydrates into glucose. It is primarily used in diabetes mellitus type 2 for establishing greater glycemic control by preventing the digestion of carbohydrates into monosaccharides which can be absorbed by the body.

Acetylcholinesterase, also known as AChE or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and of some other choline esters that function as neurotransmitters. AChE is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate synaptic transmission. It belongs to carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides.

Neutral alpha-glucosidase AB is an enzyme that in humans is encoded by the GANAB gene.

4-Aminoquinoline is a form of aminoquinoline with the amino group at the 4-position of the quinoline. The compound has been used as a precursor for the synthesis of its derivatives.

Tenuazonic acid is a mycotoxin produced by Alternaria species. It is a powerful eukaryotic protein synthesis inhibitor. It is a tetrameric acid that is ubiquitous in biological environments and prevents the release of newly synthesized protein from the ribosome. Its toxicity is the highest among all Alternaria mycotoxins and has both phytotoxic and cytotoxic properties. In 1991 Tenuazonic acid was reported to inhibit skin tumor promotion in mice.

Typha domingensis, known commonly as southern cattail or cumbungi, is a perennial herbaceous plant of the genus Typha.
Glucan endo-1,3-beta-D-glucosidase is an enzyme with systematic name 3-beta-D-glucan glucanohydrolase. This enzyme catalyses the following chemical reaction
Glucan endo-1,3-alpha-glucosidase is an enzyme with systematic name 3-alpha-D-glucan 3-glucanohydrolase. The enzyme catalyses the following chemical reaction
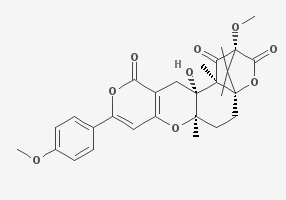
Terreulactone A is a meroterpenoid isolate of Aspergillus with anti-acetylcholinesterase activity.
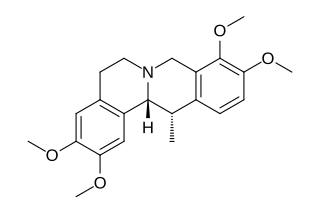
Corydaline is an acetylcholinesterase inhibitor isolated from Corydalis yanhusuo.
Medicinal fungi are fungi which contain metabolites or can be induced to produce metabolites through biotechnology to develop prescription drugs. Compounds successfully developed into drugs or under research include antibiotics, anti-cancer drugs, cholesterol and ergosterol synthesis inhibitors, psychotropic drugs, immunosuppressants and fungicides.
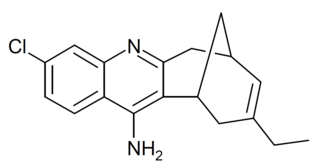
Huprine X is a synthetic cholinergic compound developed as a hybrid between the natural product Huperzine A and the synthetic drug tacrine. It is one of the most potent reversible inhibitors of acetylcholinesterase known, with a binding affinity of 0.026nM, as well as showing direct agonist activity at both nicotinic and muscarinic acetylcholine receptors. In animal studies it has nootropic and neuroprotective effects, and is used in research into Alzheimer's disease, and although huprine X itself has not been researched for medical use in humans, a large family of related derivatives have been developed.
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.















