
Hydrogen peroxide is a chemical compound with the formula H2O2. In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution in water for consumer use and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or "high-test peroxide", decomposes explosively when heated and has been used as both a monopropellant and an oxidizer in rocketry.
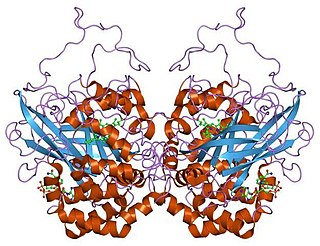
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species (ROS). Catalase has one of the highest turnover numbers of all enzymes; one catalase molecule can convert millions of hydrogen peroxide molecules to water and oxygen each second.

An echinoderm is any deuterostomal animal of the phylum Echinodermata, which includes starfish, brittle stars, sea urchins, sand dollars and sea cucumbers, as well as the sessile sea lilies or "stone lilies". While bilaterally symmetrical as larvae, as adults echinoderms are recognisable by their usually five-pointed radial symmetry, and are found on the sea bed at every ocean depth from the intertidal zone to the abyssal zone. The phylum contains about 7,600 living species, making it the second-largest group of deuterostomes after the chordates, as well as the largest marine-only phylum. The first definitive echinoderms appeared near the start of the Cambrian.
In chemistry, a disulfide is a compound containing a R−S−S−R′ functional group or the S2−
2 anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.

Sea urchins or urchins are typically spiny, globular animals, echinoderms in the class Echinoidea. About 950 species live on the seabed, inhabiting all oceans and depth zones from the intertidal to 5,000 metres. Their tests are round and spiny, typically from 3 to 10 cm across. Sea urchins move slowly, crawling with their tube feet, and sometimes pushing themselves with their spines. They feed primarily on algae but also eat slow-moving or sessile animals. Their predators include sea otters, starfish, wolf eels, and triggerfish.

Glutathione is an organic compound with the chemical formula HOCOCH(NH2)CH2CH2CONHCH(CH2SH)CONHCH2COOH. It is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine.

Hydrogen peroxide–urea is a white crystalline solid chemical compound composed of equal amounts of hydrogen peroxide and urea. It contains solid and water-free hydrogen peroxide, which offers a higher stability and better controllability than liquid hydrogen peroxide when used as an oxidizing agent. Often called carbamide peroxide in dentistry, it is used as a source of hydrogen peroxide when dissolved in water for bleaching, disinfection and oxidation.

Trypanothione is an unusual form of glutathione containing two molecules of glutathione joined by a spermidine (polyamine) linker. It is found in parasitic protozoa such as leishmania and trypanosomes. These protozoal parasites are the cause of leishmaniasis, sleeping sickness and Chagas' disease. Trypanothione was discovered by Alan Fairlamb. Its structure was proven by chemical synthesis. It is present mainly in the Kinetoplastida but can be found in other parasitic protozoa such as Entamoeba histolytica. Since this thiol is absent from humans and is essential for the survival of the parasites, the enzymes that make and use this molecule are targets for the development of new drugs to treat these diseases.
Lipid peroxidation, or lipid oxidation, is a complex chemical process that leads to oxidative degradation of lipids, resulting in the formation of peroxide and hydroperoxide derivatives. It occurs when free radicals, specifically reactive oxygen species (ROS), interact with lipids within cell membranes, typically polyunsaturated fatty acids (PUFAs) as they have carbon–carbon double bonds. This reaction leads to the formation of lipid radicals, collectively referred to as lipid peroxides or lipid oxidation products (LOPs), which in turn react with other oxidizing agents, leading to a chain reaction that results in oxidative stress and cell damage.
Respiratory burst is the rapid release of the reactive oxygen species (ROS), superoxide anion and hydrogen peroxide, from different cell types.
External fertilization is a mode of reproduction in which a male organism's sperm fertilizes a female organism's egg outside of the female's body. It is contrasted with internal fertilization, in which sperm are introduced via insemination and then combine with an egg inside the body of a female organism.
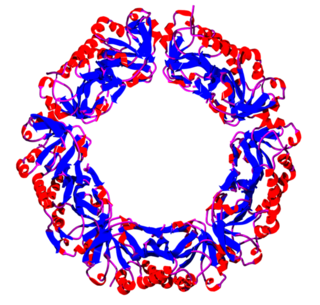
Peroxiredoxins are a ubiquitous family of antioxidant enzymes that also control cytokine-induced peroxide levels and thereby mediate signal transduction in mammalian cells. The family members in humans are PRDX1, PRDX2, PRDX3, PRDX4, PRDX5, and PRDX6. The physiological importance of peroxiredoxins is indicated by their relative abundance. Their function is the reduction of peroxides, specifically hydrogen peroxide, alkyl hydroperoxides, and peroxynitrite.

Heterosigma akashiwo is a species of microscopic algae of the class Raphidophyceae. It is a swimming marine alga that episodically forms toxic surface aggregations known as harmful algal bloom. The species name akashiwo is from the Japanese for "red tide".

Pycnopodia helianthoides, commonly known as the sunflower sea star, is a large sea star found in the northeastern Pacific Ocean. The only species of its genus, it is among the largest sea stars in the world, with a maximum arm span of 1 m (3.3 ft). Adult sunflower sea stars usually have 16 to 24 limbs. They vary in color. A carnivorous animal, Sunflower sea stars eat many different kinds of dead and alive prey to fill their diets. They are predatory, feeding mostly on sea urchins, clams, sea snails, and other small invertebrates. Although the species was widely distributed throughout the northeast Pacific, its population rapidly declined from 2013. The sunflower sea star is classified as Critically Endangered on the IUCN Red List.

Strongylocentrotus purpuratus is a species of sea urchin in the family Strongylocentrotidae commonly known as the purple sea urchin. It lives along the eastern edge of the Pacific Ocean extending from Ensenada, Mexico, to British Columbia, Canada. This sea urchin species is deep purple in color, and lives in lower inter-tidal and nearshore sub-tidal communities. Its eggs are orange when secreted in water. January, February, and March function as the typical active reproductive months for the species. Sexual maturity is reached around two years. It normally grows to a diameter of about 10 cm (4 inches) and may live as long as 70 years.

Vaginal flora, vaginal microbiota or vaginal microbiome are the microorganisms that colonize the vagina. They were discovered by the German gynecologist Albert Döderlein in 1892 and are part of the overall human flora. The amount and type of bacteria present have significant implications for an individual's overall health. The primary colonizing bacteria of a healthy individual are of the genus Lactobacillus, such as L. crispatus, and the lactic acid they produce is thought to protect against infection by pathogenic species.

Spinochrome E is a polyhydroxylated 1,4-naphthoquinone pigment found in sea urchin shell ("test"), spine, gonads, coelomic fluid, and eggs, of sea urchin commonly known as spinochromes. These natural phenolic compounds are quinones with potential pharmacological properties. The several hydroxyl groups are appropriate for free-radical scavenging, which diminishes ROS and prevents redox imbalance. Mechanisms are described such as scavenging of reactive oxygen species (ROS), interaction with lipid peroxide radicals, chelation of metal ions, inhibition of lipid peroxidation and regulation of the cell redox potential.
All living cells produce reactive oxygen species (ROS) as a byproduct of metabolism. ROS are reduced oxygen intermediates that include the superoxide radical (O2−) and the hydroxyl radical (OH•), as well as the non-radical species hydrogen peroxide (H2O2). These ROS are important in the normal functioning of cells, playing a role in signal transduction and the expression of transcription factors. However, when present in excess, ROS can cause damage to proteins, lipids and DNA by reacting with these biomolecules to modify or destroy their intended function. As an example, the occurrence of ROS have been linked to the aging process in humans, as well as several other diseases including Alzheimer's, rheumatoid arthritis, Parkinson's, and some cancers. Their potential for damage also makes reactive oxygen species useful in direct protection from invading pathogens, as a defense response to physical injury, and as a mechanism for stopping the spread of bacteria and viruses by inducing programmed cell death.
Mycothiol synthase is an enzyme with systematic name acetyl-CoA:desacetylmycothiol O-acetyltransferase. This enzyme catalyses the following chemical reaction
Maria Byrne is an Australian marine biologist, and professor of marine and developmental biology at the University of Sydney and a member of the Sydney Environment Institute. She spent 12 years as director of the university's research station on One Tree Island.













