Related Research Articles

The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae, and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa.

The endomembrane system is composed of the different membranes (endomembranes) that are suspended in the cytoplasm within a eukaryotic cell. These membranes divide the cell into functional and structural compartments, or organelles. In eukaryotes the organelles of the endomembrane system include: the nuclear membrane, the endoplasmic reticulum, the Golgi apparatus, lysosomes, vesicles, endosomes, and plasma (cell) membrane among others. The system is defined more accurately as the set of membranes that forms a single functional and developmental unit, either being connected directly, or exchanging material through vesicle transport. Importantly, the endomembrane system does not include the membranes of plastids or mitochondria, but might have evolved partially from the actions of the latter.

The Golgi apparatus, also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus.
The Coat Protein Complex II, or COPII, is a group of proteins that facilitate the formation of vesicles to transport proteins from the endoplasmic reticulum to the Golgi apparatus or endoplasmic-reticulum–Golgi intermediate compartment. This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI complex. COPII is assembled in two parts: first an inner layer of Sar1, Sec23, and Sec24 forms; then the inner coat is surrounded by an outer lattice of Sec13 and Sec31.

COPI is a coatomer, a protein complex that coats vesicles transporting proteins from the cis end of the Golgi complex back to the rough endoplasmic reticulum (ER), where they were originally synthesized, and between Golgi compartments. This type of transport is retrograde transport, in contrast to the anterograde transport associated with the COPII protein. The name "COPI" refers to the specific coat protein complex that initiates the budding process on the cis-Golgi membrane. The coat consists of large protein subcomplexes that are made of seven different protein subunits, namely α, β, β', γ, δ, ε and ζ.
A secretory protein is any protein, whether it be endocrine or exocrine, which is secreted by a cell. Secretory proteins include many hormones, enzymes, toxins, and antimicrobial peptides. Secretory proteins are synthesized in the endoplasmic reticulum.
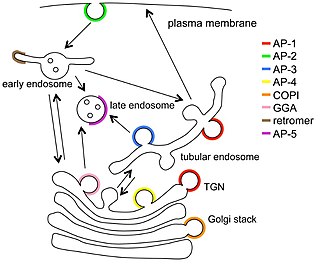
Vesicular transport adaptor proteins are proteins involved in forming complexes that function in the trafficking of molecules from one subcellular location to another. These complexes concentrate the correct cargo molecules in vesicles that bud or extrude off of one organelle and travel to another location, where the cargo is delivered. While some of the details of how these adaptor proteins achieve their trafficking specificity has been worked out, there is still much to be learned.

The vesicular-tubular cluster (VTC), also referred to as the endoplasmic-reticulum–Golgi intermediate compartment (ERGIC), is an organelle in eukaryotic cells. This compartment mediates trafficking between the endoplasmic reticulum (ER) and Golgi complex, facilitating the sorting of cargo. The cluster was first identified in 1988 using an antibody to the protein that has since been named ERGIC-53.
The coatomer is a protein complex that coats membrane-bound transport vesicles. Two types of coatomers are known:

Endoplasmic-reticulum-associated protein degradation (ERAD) designates a cellular pathway which targets misfolded proteins of the endoplasmic reticulum for ubiquitination and subsequent degradation by a protein-degrading complex, called the proteasome.

KDEL (Lys-Asp-Glu-Leu) endoplasmic reticulum protein retention receptor 1, also known as KDELR1, is a protein which in humans is encoded by the KDELR1 gene.

Surfeit locus protein 4 or Surf4 is a protein involved in regulating export of some proteins from the endoplasmic reticulum to the golgi bodies. Surf4 is involved in trafficking soluble proteins, namely lipoproteins and PCSK9. It recognizes cargo proteins via a three-amino-acid sequence near the N-termini. The related protein in yeast is called Erv29p.

SEC23-interacting protein is a protein that in humans is encoded by the SEC23IP gene.
KDEL is a target peptide sequence in mammals and plants located on the C-terminal end of the amino acid structure of a protein. The KDEL sequence prevents a protein from being secreted from the endoplasmic reticulum (ER) and facilitates its return if it is accidentally exported.
Coat protein may refer to:

Sec14 is a cytosolic protein found in yeast which plays a role in the regulation of several cellular functions, specifically those related to intracellular transport. Encoded by the Sec14 gene, Sec14p may transport phosphatidylinositol and phosphatidylcholine produced in the endoplasmic reticulum and the Golgi body to other cellular membranes. Additionally, Sec14p potentially plays a role in the localization of lipid raft proteins. Sec14p is an essential gene in yeast, and is homologous in function to phosphatidylinositol transfer protein in mammals. A conditional mutant with non-functional Sec14p presents with Berkeley bodies and deficiencies in protein secretion.
SEC31 is a protein which in yeast promotes the formation of COPII transport vesicles from the Endoplasmic Reticulum (ER). The coat has two main functions, the physical deformation of the endoplasmic reticulum membrane into vesicles and the selection of cargo molecules.
KKXX and for some proteins XKXX is a target peptide motif located in the C terminus in the amino acid structure of a protein responsible for retrieval of endoplasmic reticulum (ER) membrane proteins to and from the Golgi apparatus. These ER membrane proteins are transmembrane proteins that are then embedded into the ER membrane after transport from the Golgi. This motif is exclusively cytoplasmic and interacts with the COPI protein complex to target the ER from the cis end of the Golgi apparatus by retrograde transport.

Transmembrane emp24 domain-containing protein 5 is a protein that in humans is encoded by the TMED5 gene.
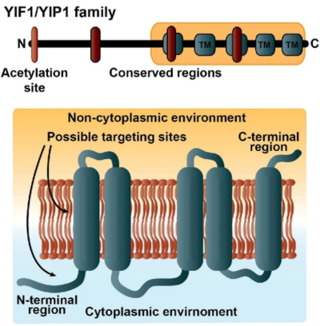
The Yip1 domain family is a group of proteins involved in regulating secretory traffic in eukaryotes. The family consists of four members in yeast and nine members in humans. Family members have a shared architecture containing five transmembrane domains.
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Pastor-Cantizano N, Montesinos JC, Bernat-Silvestre C, Marcote MJ, Aniento F (July 2016). "p24 family proteins: key players in the regulation of trafficking along the secretory pathway". Protoplasma. 253 (4): 967–85. doi:10.1007/s00709-015-0858-6. PMID 26224213. S2CID 254088111.