
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform is an enzyme that is encoded by the PPP2CA gene.

14-3-3 protein gamma is a protein that in humans is encoded by the YWHAG gene.

Serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A alpha isoform is an enzyme that in humans is encoded by the PPP2R1A gene. In the plant Arabidopsis thaliana a similar enzyme is encoded by the RCN1 gene (At1g25490).

Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B beta isoform is an enzyme that in humans is encoded by the PPP2R2B gene.

Serine/threonine-protein phosphatase 2A catalytic subunit beta isoform is an enzyme that in humans is encoded by the PPP2CB gene.

Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit alpha isoform is an enzyme that in humans is encoded by the PPP2R5A gene.

Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit gamma isoform is an enzyme that in humans is encoded by the PPP2R5C gene.

Serine/threonine-protein phosphatase 2A regulatory subunit B is an enzyme that in humans is encoded by the PPP2R4 gene.

Serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A beta isoform is an enzyme that in humans is encoded by the PPP2R1B gene.

Serine/threonine-protein phosphatase 2A regulatory subunit B'' subunit alpha is an enzyme that in humans is encoded by the PPP2R3A gene. Protein phosphatase 2 is one of the four major Ser/Thr phosphatases and is implicated in the negative control of cell growth and division. Protein phosphatase 2 holoenzymes are heterotrimeric proteins composed of a structural subunit A, a catalytic subunit C, and a regulatory subunit B. The regulatory subunit is encoded by a diverse set of genes that have been grouped into the B/PR55, B'/PR61, and B''/PR72 families. These different regulatory subunits confer distinct enzymatic specificities and intracellular localizations to the holozenzyme. The product of this gene belongs to the B'' family. The B'' family has been further divided into subfamilies. The product of this gene belongs to the alpha subfamily of regulatory subunit B''. Alternative splicing results in multiple transcript variants encoding different isoforms.

Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit delta isoform is an enzyme that in humans is encoded by the PPP2R5D gene. Mutations in PPP2R5D cause Jordan's Syndrome.

Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B gamma isoform is an enzyme that in humans is encoded by the PPP2R2C gene.

Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit beta isoform is an enzyme that in humans is encoded by the PPP2R5B gene.

Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit epsilon isoform is an enzyme that in humans is encoded by the PPP2R5E gene.
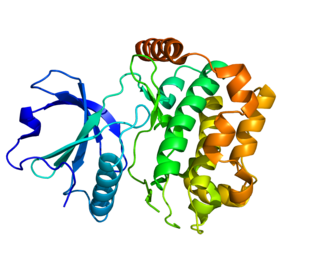
Serine/threonine-protein kinase 24 is an enzyme that in humans is encoded by the STK24 gene located in the chromosome 13, band q32.2. It is also known as Mammalian STE20-like protein kinase 3 (MST-3). The protein is 443 amino acids long and its mass is 49 kDa.

Serine/threonine-protein phosphatase 2A regulatory subunit B'' subunit beta is an enzyme that in humans is encoded by the PPP2R3B gene.

Protein phosphatase 2 (PP2), also known as PP2A, is an enzyme that in humans is encoded by the PPP2CA gene. The PP2A heterotrimeric protein phosphatase is ubiquitously expressed, accounting for a large fraction of phosphatase activity in eukaryotic cells. Its serine/threonine phosphatase activity has a broad substrate specificity and diverse cellular functions. Among the targets of PP2A are proteins of oncogenic signaling cascades, such as Raf, MEK, and AKT, where PP2A may act as a tumor suppressor.

Protein phosphatase 1 (PP1) belongs to a certain class of phosphatases known as protein serine/threonine phosphatases. This type of phosphatase includes metal-dependent protein phosphatases (PPMs) and aspartate-based phosphatases. PP1 has been found to be important in the control of glycogen metabolism, muscle contraction, cell progression, neuronal activities, splicing of RNA, mitosis, cell division, apoptosis, protein synthesis, and regulation of membrane receptors and channels.
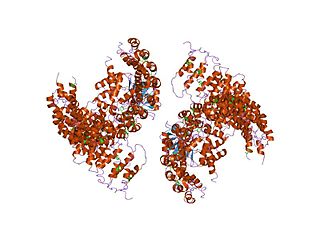
Protein phosphatase 2A (PP2A) is a major intracellular protein phosphatase that regulates multiple aspects of cell growth and metabolism. Phosphorylation enables the activation or The ability of this widely distributed heterotrimeric enzyme to act on a diverse array of substrates is largely controlled by the nature of its regulatory B subunit. There are multiple families of B subunits, this family is called the B56 family.
Ceramide-activated protein phosphatases (CAPPs) are a group of enzymes that are activated by the lipid second messenger ceramide. Known CAPPs include members of the protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) families. CAPPs are a subset of intracellular serine/threonine phosphatases. Each CAPP consists of a catalytic subunit which confers phosphatase activity and a regulatory subunit which confers substrate specificity. CAPP involvement has been implicated in glycogen metabolism, apoptotic pathways related to cancer and other cellular pathways related to Alzheimer’s disease.





















