In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula CH3. In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond, it can be found on its own in any of three forms: methanide anion, methylium cation or methyl radical. The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed.

Hepatitis is inflammation of the liver tissue. Some people or animals with hepatitis have no symptoms, whereas others develop yellow discoloration of the skin and whites of the eyes (jaundice), poor appetite, vomiting, tiredness, abdominal pain, and diarrhea. Hepatitis is acute if it resolves within six months, and chronic if it lasts longer than six months. Acute hepatitis can resolve on its own, progress to chronic hepatitis, or (rarely) result in acute liver failure. Chronic hepatitis may progress to scarring of the liver (cirrhosis), liver failure, and liver cancer.
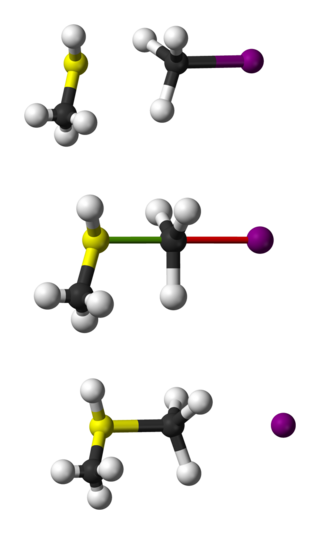
The bimolecular nucleophilic substitution (SN2) is a type of reaction mechanism that is common in organic chemistry. In the SN2 reaction, a strong nucleophile forms a new bond to an sp3-hybridised carbon atom via a backside attack, all while the leaving group detaches from the reaction center in a concerted fashion.

Cerebral edema is excess accumulation of fluid (edema) in the intracellular or extracellular spaces of the brain. This typically causes impaired nerve function, increased pressure within the skull, and can eventually lead to direct compression of brain tissue and blood vessels. Symptoms vary based on the location and extent of edema and generally include headaches, nausea, vomiting, seizures, drowsiness, visual disturbances, dizziness, and in severe cases, death.
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons.
In organic chemistry, a carbanion is an anion in which carbon is negatively charged.

The thiazolidinediones, abbreviated as TZD, also known as glitazones after the prototypical drug ciglitazone, are a class of heterocyclic compounds consisting of a five-membered C3NS ring. The term usually refers to a family of drugs used in the treatment of diabetes mellitus type 2 that were introduced in the late 1990s. As of 2024, there are two FDA-approved drugs in this class, pioglitazone and rosiglitazone.

Rosiglitazone is an antidiabetic drug in the thiazolidinedione class. It works as an insulin sensitizer, by binding to the PPAR in fat cells and making the cells more responsive to insulin. It is marketed by the pharmaceutical company GlaxoSmithKline (GSK) as a stand-alone drug or for use in combination with metformin or with glimepiride. First released in 1999, annual sales peaked at approximately $2.5-billion in 2006; however, following a meta-analysis in 2007 that linked the drug's use to an increased risk of heart attack, sales plummeted to just $9.5-million in 2012. The drug's patent expired in 2012.

Pioglitazone, sold under the brand name Actos among others, is an anti-diabetic medication used to treat type 2 diabetes. It may be used with metformin, a sulfonylurea, or insulin. Use is recommended together with exercise and diet. It is not recommended in type 1 diabetes. It is taken by mouth.
Hydrogen–deuterium exchange is a chemical reaction in which a covalently bonded hydrogen atom is replaced by a deuterium atom, or vice versa. It can be applied most easily to exchangeable protons and deuterons, where such a transformation occurs in the presence of a suitable deuterium source, without any catalyst. The use of acid, base or metal catalysts, coupled with conditions of increased temperature and pressure, can facilitate the exchange of non-exchangeable hydrogen atoms, so long as the substrate is robust to the conditions and reagents employed. This often results in perdeuteration: hydrogen-deuterium exchange of all non-exchangeable hydrogen atoms in a molecule.

Metabolic dysfunction–associated steatotic liver disease (MASLD), previously known as non-alcoholic fatty liver disease (NAFLD), is a type of chronic liver disease. This condition is diagnosed when there is excessive fat build-up in the liver, and at least one metabolic risk factor. When there is also increased alcohol intake, the term MetALD, or metabolic dysfunction and alcohol associated/related liver disease is used, and differentiated from alcohol-related liver disease (ALD) where alcohol is the predominant cause of the steatotic liver disease. The terms non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis have been used to describe different severities, the latter indicating the presence of further liver inflammation. NAFL is less dangerous than NASH and usually does not progress to it, but this progression may eventually lead to complications, such as cirrhosis, liver cancer, liver failure, and cardiovascular disease.
2-Oxazolidinone is a heterocyclic organic compound containing both nitrogen and oxygen in a 5-membered ring.

Muraglitazar is a dual peroxisome proliferator-activated receptor agonist with affinity to PPARα and PPARγ.

Remogliflozin etabonate (INN/USAN) is a drug of the gliflozin class for the treatment of non-alcoholic steatohepatitis ("NASH") and type 2 diabetes. Remogliflozin was discovered by the Japanese company Kissei Pharmaceutical and is currently being developed by BHV Pharma, a wholly owned subsidiary of North Carolina, US-based Avolynt, and Glenmark Pharmaceuticals through a collaboration with BHV. In 2002, GlaxoSmithKline (GSK) received a license to use it. From 2002 to 2009, GSK carried out a significant clinical development program for the treatment of type-2 diabetes mellitus in various nations across the world and obesity in the UK. Remogliflozin etabonate's pharmacokinetics, pharmacodynamics, and clinical dose regimens were characterized in 18 Phase I and 2 Phase II investigations. Due to financial concerns, GSK stopped working on remogliflozin and sergliflozin, two further SGLT2 inhibitors that were licensed to the company, in 2009. Remogliflozin was commercially launched first in India by Glenmark in May 2019.

Saroglitazar is a drug for the treatment of type 2 diabetes mellitus, dyslipidemia, NASH and NAFLD It is approved for use in India by the Drug Controller General of India. Saroglitazar is indicated for the treatment of diabetic dyslipidemia and hypertriglyceridemia with type 2 diabetes mellitus not controlled by statin therapy. In clinical studies, saroglitazar has demonstrated reduction of triglycerides (TG), LDL cholesterol, VLDL cholesterol, non-HDL cholesterol and an increase in HDL cholesterol a characteristic hallmark of atherogenic diabetic dyslipidemia (ADD). It has also shown anti-diabetic medication properties by reducing the fasting plasma glucose and HBA1c in diabetes patients.

A deuterated drug is a small molecule medicinal product in which one or more of the hydrogen atoms in the drug molecule have been replaced by its heavier stable isotope deuterium. Because of the kinetic isotope effect, deuterium-containing drugs may have significantly lower rates of metabolism, and hence a longer half-life.

This is a list of women chemists. It should include those who have been important to the development or practice of chemistry. Their research or application has made significant contributions in the area of basic or applied chemistry.

Bromochlorofluoromethane or fluorochlorobromomethane, is a chemical compound and trihalomethane derivative with the chemical formula CHBrClF. As one of the simplest possible stable chiral compounds, it is useful for fundamental research into this area of chemistry. However, its relative instability to hydrolysis, and lack of suitable functional groups, made separation of the enantiomers of bromochlorofluoromethane especially challenging, and this was not accomplished until almost a century after it was first synthesised, in March 2005, though it has now been done by a variety of methods. More recent research using bromochlorofluoromethane has focused on its potential use for experimental measurement of parity violation, a major unsolved problem in quantum physics.
Anthony William Czarnik is an American chemist and inventor. He is best known for pioneering studies in the field of fluorescent chemosensors and co-founding Illumina, Inc., a biotechnology company in San Diego. Czarnik was also the founding editor of ACS Combinatorial Science. He currently serves as an adjunct visiting professor at the University of Nevada, Reno.
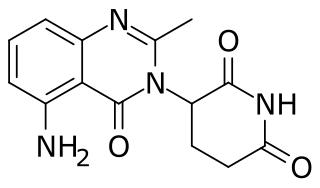
Avadomide is an experimental cereblon E3 ligase modulator, or thalidomide analog studied to see if it is effective against cancer.














