Related Research Articles
An antiemetic is a drug that is effective against vomiting and nausea. Antiemetics are typically used to treat motion sickness and the side effects of opioid analgesics, general anaesthetics, and chemotherapy directed against cancer. They may be used for severe cases of gastroenteritis, especially if the patient is dehydrated.

Granisetron is a serotonin 5-HT3 receptor antagonist used as an antiemetic to treat nausea and vomiting following chemotherapy and radiotherapy. Its main effect is to reduce the activity of the vagus nerve, which is a nerve that activates the vomiting center in the medulla oblongata. It does not have much effect on vomiting due to motion sickness. This drug does not have any effect on dopamine receptors or muscarinic receptors.
Postoperative nausea and vomiting (PONV) is the phenomenon of nausea, vomiting, or retching experienced by a patient in the post-anesthesia care unit (PACU) or within 24 hours following a surgical procedure. PONV affects about 10% of the population undergoing general anaesthesia each year. PONV can be unpleasant and lead to a delay in mobilization and food, fluid, and medication intake following surgery.
The chemoreceptor trigger zone (CTZ) is an area of the medulla oblongata that receives inputs from blood-borne drugs or hormones, and communicates with other structures in the vomiting center to initiate vomiting. The CTZ is located within the area postrema, which is on the floor of the fourth ventricle and is outside of the blood–brain barrier. It is also part of the vomiting center itself. The neurotransmitters implicated in the control of nausea and vomiting include acetylcholine, dopamine, histamine, substance P, and serotonin. There are also opioid receptors present, which may be involved in the mechanism by which opiates cause nausea and vomiting. The blood–brain barrier is not as developed here; therefore, drugs such as dopamine which cannot normally enter the CNS may still stimulate the CTZ.

Aprepitant, sold under the brand name Emend among others, is a medication used to prevent chemotherapy-induced nausea and vomiting (CINV) and to prevent postoperative nausea and vomiting (PONV). It may be used together with ondansetron and dexamethasone. It is taken by mouth or administered by intravenous injection.

Dolasetron (trade name Anzemet) is a serotonin 5-HT3 receptor antagonist used to treat nausea and vomiting following chemotherapy. Its main effect is to reduce the activity of the vagus nerve, which is a nerve that activates the vomiting center in the medulla oblongata. It does not have much antiemetic effect when symptoms are due to motion sickness. This drug does not have any effect on dopamine receptors or muscarinic receptors.
The 5-HT3 receptor belongs to the Cys-loop superfamily of ligand-gated ion channels (LGICs) and therefore differs structurally and functionally from all other 5-HT receptors (5-hydroxytryptamine, or serotonin receptors) which are G protein-coupled receptors. This ion channel is cation-selective and mediates neuronal depolarization and excitation within the central and peripheral nervous systems.

Vomiting is the involuntary, forceful expulsion of the contents of one's stomach through the mouth and sometimes the nose.
Neurokinin 1 (NK1) antagonists (-pitants) are a novel class of medications that possesses unique antidepressant, anxiolytic, and antiemetic properties. NK-1 antagonists boost the efficacy of 5-HT3 antagonists to prevent nausea and vomiting. The discovery of neurokinin 1 (NK1) receptor antagonists was a turning point in the prevention of nausea and vomiting associated with cancer chemotherapy.

The 5-HT3 antagonists, informally known as "setrons", are a class of drugs that act as receptor antagonists at the 5-HT3 receptor, a subtype of serotonin receptor found in terminals of the vagus nerve and in certain areas of the brain. With the notable exceptions of alosetron and cilansetron, which are used in the treatment of irritable bowel syndrome, all 5-HT3 antagonists are antiemetics, used in the prevention and treatment of nausea and vomiting. They are particularly effective in controlling the nausea and vomiting produced by cancer chemotherapy and are considered the gold standard for this purpose.
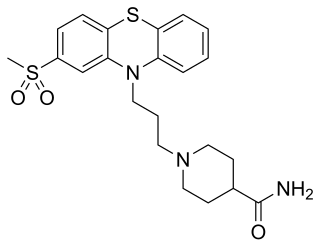
Metopimazine, sold under the brand names Vogalen and Vogalene, is an antiemetic of the phenothiazine group which is used to treat nausea and vomiting. It is marketed in Europe, Canada, and South America. As of August 2020, metopimazine has been repurposed and is additionally under development for use in the United States for the treatment of gastroparesis.
A serotonin antagonist, or serotonin receptor antagonist, is a drug used to inhibit the action of serotonin and serotonergic drugs at serotonin (5-HT) receptors.

Ricasetron (BRL-46470) is a drug which acts as a selective antagonist at the serotonin 5-HT3 receptor. It has antiemetic effects as with other 5-HT3 antagonists, and also has anxiolytic effects significantly stronger than other related drugs, and with less side effects than benzodiazepine anxiolytics. However, it has never been developed for medical use.

Dazopride (AHR-5531) is an antiemetic and gastroprokinetic agent of the benzamide class which was never marketed. It acts as a 5-HT3 receptor antagonist and 5-HT4 receptor agonist. In addition to its gastrointestinal effects, dazopride facilitates learning and memory in mice.
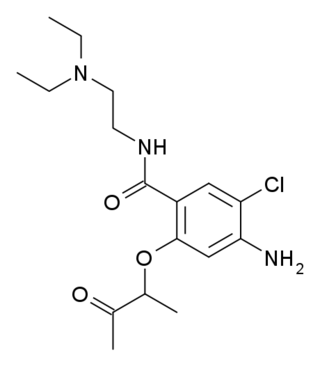
Batanopride (BMY-25,801) is an antiemetic drug of the benzamide class which acts as a selective 5-HT3 receptor antagonist. It was trialled to reduce nausea during cancer chemotherapy, but was never approved for medical use due to dose-limiting side effects including hypotension and long QT syndrome.
Chemotherapy-induced nausea and vomiting (CINV) is a common side-effect of many cancer treatments. Nausea and vomiting are two of the most feared cancer treatment-related side effects for cancer patients and their families. In 1983, Coates et al. found that patients receiving chemotherapy ranked nausea and vomiting as the first and second most severe side effects, respectively. Up to 20% of patients receiving highly emetogenic agents in this era postponed, or even refused, potentially curative treatments. Since the 1990s, several novel classes of antiemetics have been developed and commercialized, becoming a nearly universal standard in chemotherapy regimens, and helping to better manage these symptoms in a large portion of patients. Efficient mediation of these unpleasant and sometimes debilitating symptoms results in increased quality of life for the patient, and better overall health of the patient, and, due to better patient tolerance, more effective treatment cycles.

Cancer and nausea are associated in about fifty percent of people affected by cancer. This may be as a result of the cancer itself, or as an effect of the treatment such as chemotherapy, radiation therapy, or other medication such as opiates used for pain relief. About 70 to 80% of people undergoing chemotherapy experience nausea or vomiting. Nausea and vomiting may also occur in people not receiving treatment, often as a result of the disease involving the gastrointestinal tract, electrolyte imbalance, or as a result of anxiety. Nausea and vomiting may be experienced as the most unpleasant side effects of cytotoxic drugs and may result in patients delaying or refusing further radiotherapy or chemotherapy.

Netupitant is an antiemetic medication. In the United States, the combinations of netupitant/palonosetron and the prodrug fosnetupitant/palonosetron are approved by the Food and Drug Administration for the prevention of acute and delayed chemotherapy-induced nausea and vomiting, including highly emetogenic chemotherapy such as with cisplatin. In the European Union, the combinations are approved by the European Medicines Agency (EMA) for the same indication.

Rolapitant (INN, trade name Varubivə-ROO-bee in the US and Varuby in the European Union) is a drug originally developed by Schering-Plough and licensed for clinical development by Tesaro, which acts as a selective NK1 receptor antagonist (antagonist for the NK1 receptor). It has been approved as a medication for the treatment of chemotherapy-induced nausea and vomiting (CINV) after clinical trials showed it to have similar or improved efficacy and some improvement in safety over existing drugs for this application.

Indisetron is a drug used for prophylaxis of chemotherapy-induced nausea and vomiting. It was approved by Japan's Pharmaceuticals and Medical Devices Agency in 2004.
References
- 1 2 Sanger, G.J., Andrews, P.L.R. (2018) "A history of drug discovery for treatment of nausea and vomiting and the implications for future research". Frontiers in Pharmacology9, 913. doi: 10.3389/fphar.2018.00913
- ↑ Daphne Christie; Tilli Tansey, eds. (2007) "The Discovery, Use and Impact of Platinum Salts as Chemotherapy Agents for Cancer", Wellcome Witnesses to Contemporary Medicine, History of Modern Biomedicine Research Group, ISBN 978-0-85484-112-7.
- ↑ P. L. R. Andrews & J. Z. Young (1993)“Gastric motility patterns for digestion and vomiting evoked by sympathetic nerve stimulation and 5-hydroxytryptamine in the dogfish Scyliorhinus canicula” Philosophical Transactions of the Royal Society B 342.
- ↑ Andrews, P. L. R., Rapeport, W. G., and Sanger, G. J. (1988) "Neuropharmacology of emesis induced by anti-cancer therapy" Trends in Pharmacological Sciences9, 334–341. doi: 10.1016/0165-6147(88)90106-X
- 1 2 Watson, J.W., Gonsalves, S.F., Fossa, A.A., McLean, S., Seeger, T., Obach, S., Andrews, P. L. R. (1995) "The anti-emetic effects of CP-99, 994 in the ferret and the dog: role of the NK1 receptor" British Journal of Pharmacology115 84–94.
- ↑ Miner, W.D. and Sanger, G.J. (1986) "Inhibition of cisplatin-induced vomiting by selective 5-hydroxytryptamine M-receptor antagonism" British Journal of Pharmacology88 497–499.
- ↑ Bermudez, J., Boyle, E. A., Miner,W. D., and Sanger, G. J. (1988). "The anti-emetic potential of the 5-hydroxytryptamine3 receptor antagonist BRL 43694". British Journal of Cancer58, 644–650.
- ↑ Currow, D. C., Coughlan, M., Fardell, B., and Cooney, N. J. (1997). [https://www.jpsmjournal.com/article/S0885-3924(97)00079-1/pdf "Use of ondansetron in palliative medicine"] Journal of Pain and Symptom Management13, 302–307.
- ↑ Warr, D., and DeAngelis, C. (2009). "Controlling nausea and vomiting in patients undergoing chemotherapy. Toward more effective clinical practice" Oncology Exchange8, 23–27.
- ↑ Multinational Association for the Support of Cancer Care (MASCC). "MASCC/ESMO Antiemetic Guidelines".
- ↑ Herrstedt, J., Roila, F.,Warr, D., Celio, L., Navari, R.M., Hesketh, P.J., Chan, A., Aapro, M.S. (2017). "Updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following high emetic risk chemotherapy" Support Care Cancer25, 277–288.