Related Research Articles

Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer. This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia. It is often used together with other chemotherapy agents. Doxorubicin is given by injection into a vein.

Asparaginase is an enzyme that is used as a medication and in food manufacturing. As a medication, L-asparaginase is used to treat acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL). It is given by injection into a vein, muscle, or under the skin. A pegylated version is also available. In food manufacturing it is used to decrease acrylamide.

Toremifene, sold under the brand name Fareston among others, is a medication which is used in the treatment of advanced breast cancer in postmenopausal women. It is taken by mouth.

Ascomycin, also called Immunomycin, FR-900520, FK520, is an ethyl analog of tacrolimus (FK506) with strong immunosuppressant properties. It has been researched for the treatment of autoimmune diseases and skin diseases, and to prevent rejection after an organ transplant.
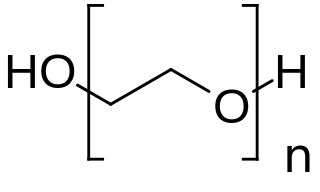
PEGylation is the process of both covalent and non-covalent attachment or amalgamation of polyethylene glycol polymer chains to molecules and macrostructures, such as a drug, therapeutic protein or vesicle, which is then described as PEGylated. PEGylation affects the resulting derivatives or aggregates interactions, which typically slows down their coalescence and degradation as well as elimination in vivo.

Lasofoxifene, sold under the brand name Fablyn, is a nonsteroidal selective estrogen receptor modulator (SERM) which is marketed by Pfizer in Lithuania and Portugal for the prevention and treatment of osteoporosis and for the treatment of vaginal atrophy, and the result of an exclusive research collaboration with Ligand Pharmaceuticals (LGND). It also appears to have had a statistically significant effect of reducing breast cancer in women according to a study published in The Journal of the National Cancer Institute.
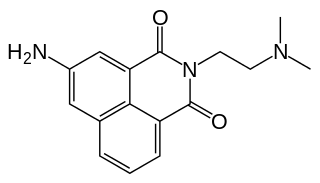
Amonafide was a drug that was being studied in the treatment of cancer. It belongs to a novel family of chemotherapeutic drugs called Naphthalimides and is a potential topoisomerase inhibitor and DNA intercalator.
Argininosuccinate synthetase is an enzyme that in humans is encoded by the ASS1 gene.

Salsalate is a medication that belongs to the salicylate and nonsteroidal anti-inflammatory drug (NSAID) classes.

Cethromycin, trade name Restanza is a ketolide antibiotic undergoing research for the treatment of community acquired pneumonia (CAP) and for the prevention of post-exposure inhalational anthrax, and was given an "orphan drug" status for this indication. Originally discovered and developed by Abbott, it was acquired by Advanced Life Sciences Inc. for further development.

ELB-139 (LS-191,811) is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.

Dexelvucitabine is a failed experimental agent for the management of human immunodeficiency virus infection. It is a cytidine nucleoside analog and nucleoside reverse transcriptase inhibitor. that inhibits HIV-1 replication in vitro. During phase II clinical trials there was some indication of a decreased mean viral load in patients with infected human immunodeficiency virus.
Substrate reduction therapy offers an approach to treatment of certain metabolic disorders, especially glycogen storage diseases and lysosomal storage disorders. In a storage disorder, a critical failure in a metabolic pathway prevents cellular breakdown and disposal of some large molecule. If residual breakdown through other pathways is insufficient to prevent harmful accumulation, the molecule accumulates in the cell and eventually interferes with normal biological processes. Examples of lysosomal storage disorders include Gaucher's disease, Tay–Sachs disease, Sandhoff disease, and Sanfilippo syndrome.
Ganitumab is a human monoclonal antibody against type 1 insulin-like growth factor receptor (IGF1R), designed for the treatment of cancers.

Fedotozine is an opioid drug which acts as a peripherally specific selective κ1-opioid receptor agonist with preference for the κ1A subtype. It was under investigation for the treatment of gastrointestinal conditions like irritable bowel syndrome and functional dyspepsia and made it to phase III clinical trials, but ultimately development was discontinued and it was never marketed.
Tovetumab is an anti-PDGFRa monoclonal antibody designed for the treatment of cancer. It was developed by MedImmune, and trialed for use in glioblastoma and non-small cell lung cancer. Development was discontinued in 2013.

Apalutamide, sold under the brand name Erleada among others, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is specifically indicated for use in conjunction with castration in the treatment of non-metastatic castration-resistant prostate cancer (NM-CRPC). It is taken by mouth.
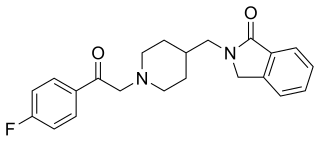
Roluperidone (former developmental code names MIN-101, CYR-101, MT-210) is a 5-HT2A and σ2 receptor antagonist under development by Minerva Neurosciences for the treatment of schizophrenia. One of its metabolites also has some affinity for the H1 receptor. Pre-clinical findings provide evidence of the effect of roluperidone on Brain-Derived Neurotrophic Factor (“BDNF”), which has been associated with neurogenesis, neuroplasticity, neuroprotection, synapse regulation, learning and memory. As of May 2018, the drug was in phase III clinical trials. In May 2020, the shares of Minerva Neurosciences plummeted 67% after the trial "failed to meet its primary endpoint of reduction in negative symptoms, and key secondary endpoints of improvement in personal and social performance measurements." However, in August 2022 Minerva submitted a New Drug Application (NDA) to the Food and Drug Administration (FDA) for the approval of roluperidone for the treatment of schizophrenia. The NDA submission in 2022 followed successful completion of a phase III clinical trial which was published in early 2022. Minerva believed that the findings of this second trial supported the claim that the drug was an effective agent for the treatment of negative symptoms in schizophrenia. However, in October 2022, FDA sent Minerva a refusal to file letter pertaining to the New Drug Application for roluperidone for treating negative symptoms in schizophrenia patients.

Mericitabine (RG-7128) is an antiviral drug, a deoxycytidine analog. It was developed as a treatment for hepatitis C, acting as a NS5B RNA polymerase inhibitor, but while it showed a good safety profile in clinical trials, it was not sufficiently effective to be used as a stand-alone agent. However mericitabine has been shown to boost the efficacy of other antiviral drugs when used alongside them, and as most modern treatment regimens for hepatitis C use a combination therapy of several antiviral drugs, clinical trials have continued to see if it can form a part of a clinically useful drug treatment program.
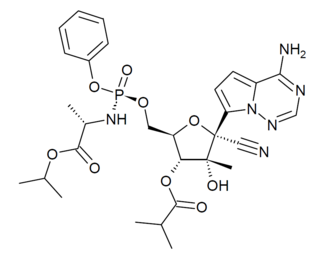
GS-6620 is an antiviral drug which is a nucleotide analogue. It was developed for the treatment of Hepatitis C but while it showed potent antiviral effects in early testing, it could not be successfully formulated into an oral dosage form due to low and variable absorption in the intestines which made blood levels unpredictable. It has however continued to be researched as a potential treatment for other viral diseases such as Ebola virus disease.
References
- 1 2 Feun L, Savaraj N (July 2006). "Pegylated arginine deiminase: a novel anticancer enzyme agent". Expert Opinion on Investigational Drugs. 15 (7): 815–22. doi:10.1517/13543784.15.7.815. PMC 3086545 . PMID 16787144.
- ↑ Kuo MT, Savaraj N, Feun LG (August 2010). "Targeted cellular metabolism for cancer chemotherapy with recombinant arginine-degrading enzymes". Oncotarget. 1 (4): 246–51. doi:10.18632/oncotarget.135. PMC 2998341 . PMID 21152246.
- ↑ Synakiewicz A, Stachowicz-Stencel T, Adamkiewicz-Drozynska E (November 2014). "The role of arginine and the modified arginine deiminase enzyme ADI-PEG 20 in cancer therapy with special emphasis on Phase I/II clinical trials". Expert Opinion on Investigational Drugs. 23 (11): 1517–29. doi:10.1517/13543784.2014.934808. PMID 24965808.
- ↑ Field GC, Pavlyk I, Szlosarek PW (February 2023). "Bench-to-Bedside Studies of Arginine Deprivation in Cancer". Molecules. 28 (5). Basel, Switzerland: 2150. doi: 10.3390/molecules28052150 . PMC 10005060 . PMID 36903394.