
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.

In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid. The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, whereas in nitrate esters, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.

Alfred Werner was a Swiss chemist who was a student at ETH Zurich and a professor at the University of Zurich. He won the Nobel Prize in Chemistry in 1913 for proposing the octahedral configuration of transition metal complexes. Werner developed the basis for modern coordination chemistry. He was the first inorganic chemist to win the Nobel Prize, and the only one prior to 1973.
In chemistry, linkage isomerism or ambidentate isomerism is a form of isomerism in which certain coordination compounds have the same composition but differ in their metal atom's connectivity to a ligand.

The cyanate ion is an anion with the chemical formula OCN−. It is a resonance of three forms: [O−−C≡N] (61%) ↔ [O=C=N−] (30%) ↔ [O+≡C−N2−] (4%).

In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia ligand. "Ammine" is spelled this way for historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.
In chemistry, hyponitrite may refer to the anion N
2O2−
2 ([ON=NO]2−), or to any ionic compound that contains it. In organic chemistry, it may also refer to the group −O−N=N−O−, or any organic compound with the generic formula R1−O−N=N−O−R2, where R1 and R2 are organic groups. Such compounds can be viewed as salts and esters of hyponitrous acid. An acid hyponitrite is an ionic compound with the anion HN
2O−
2 ([HON=NO]−).
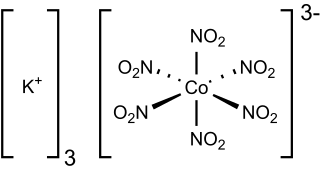
Potassium hexanitritocobaltate(III) is a salt with the formula K3[Co(NO2)6]. It is a yellow solid that is poorly soluble in water. The compound finds some use as a yellow pigment under the name Indian Yellow.

Chloropentamminecobalt chloride is the dichloride salt of the coordination complex [Co(NH3)5Cl]2+. It is a red-violet, diamagnetic, water-soluble salt. The compound has been of academic and historical interest.

Bromopentaamminecobalt(III) bromide is the dibromide salt of the cobalt coordination compound with the formula [Co(NH3)5Br]2+. It is a purple, water-soluble solid. The analogous chloropentaamminecobalt(III) chloride is also well known.
Nickel(II) nitrite is an inorganic compound with the chemical formula Ni(NO2)2. Anhydrous nickel nitrite was first discovered in 1961 by Cyril Clifford Addison, who allowed gaseous nickel tetracarbonyl to react with dinitrogen tetroxide, yielding a green smoke. Nickel nitrite was the second transition element anhydrous nitrite discovered after silver nitrite.

Sodium hyponitrite is a solid ionic compound with formula Na
2N
2O
2 or (Na+
)2[ON=NO]2−.
The nitronickelates are a class of chemical compounds containing a nickel atom complexed by nitro groups, -NO2. Nickel can be in a +2 or +3 oxidation state. There can be five (pentanitronickelates), or six, (hexanitronickelates) nitro groups per nickel atom. They can be considered the double nitrites of nickel nitrite.
Cobalt(III) chloride or cobaltic chloride is an unstable and elusive compound of cobalt and chlorine with formula CoCl
3. In this compound, the cobalt atoms have a formal charge of +3.

Nitratoauric acid, hydrogen tetranitratoaurate, or simply called gold(III) nitrate is a crystalline gold compound that forms the trihydrate, HAu(NO3)4·3H2O or more correctly H5O2Au(NO3)4·H2O. This compound is an intermediate in the process of extracting gold. In older literature it is also known as aurinitric acid.

In organometallic chemistry, transition metal complexes of nitrite describes families of coordination complexes containing one or more nitrite ligands. Although the synthetic derivatives are only of scholarly interest, metal-nitrite complexes occur in several enzymes that participate in the nitrogen cycle.
A nitrate nitrite, or nitrite nitrate, is a coordination complex or other chemical compound that contains both nitrite and nitrate anions (NO3− and NO2−). They are mixed-anion compounds, and they are mixed-valence compounds. Some have third anions. Many nitrite nitrate compounds are coordination complexes of cobalt. Such a substance was discovered by Wolcott Gibbs and Frederick Genth in 1857.
Cobalt compounds are chemical compounds formed by cobalt with other elements.













