
Streptomyces is the largest genus of Actinomycetota and the type genus of the family Streptomycetaceae. Over 500 species of Streptomyces bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, and have genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin.

Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antibiotics which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators.

Glufosinate is a naturally occurring broad-spectrum herbicide produced by several species of Streptomyces soil bacteria. Glufosinate is a non-selective, contact herbicide, with some systemic action. Plants may also metabolize bialaphos and phosalacine, other naturally occurring herbicides, directly into glufosinate. The compound irreversibly inhibits glutamine synthetase, an enzyme necessary for the production of glutamine and for ammonia detoxification, giving it antibacterial, antifungal and herbicidal properties. Application of glufosinate to plants leads to reduced glutamine and elevated ammonia levels in tissues, halting photosynthesis and resulting in plant death.

Surfactin is a cyclic lipopeptide, commonly used as an antibiotic for its capacity as a surfactant. It is an amphiphile capable of withstanding hydrophilic and hydrophobic environments. The Gram-positive bacterial species Bacillus subtilis produces surfactin for its antibiotic effects against competitors. Surfactin showcases antibacterial, antiviral, antifungal, and hemolytic effects.

Katanosins are a group of antibiotics. They are natural products with strong antibacterial potency. So far, katanosin A and katanosin B (lysobactin) have been described.

In organic chemistry, enediynes are organic compounds containing two triple bonds and one double bond.
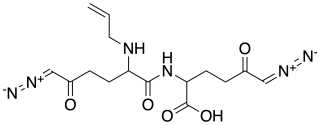
Alazopeptin is an antibiotic, with moderate anti-trypanosomal and antitumor activity. It was originally isolated from Streptacidiphilus griseoplanus, sourced from soil near Williamsburg, Iowa. It is also isolated from Kitasatospora azatica. It is still largely produced via fermentation broths of that organism. Structurally, alazopeptin is a tripeptide and contains 2 molecules of 6-diazo-5-oxo-L-norleucine and one molecule of L-alanine. In 2021 the biosynthetic pathway of alazopeptin was elucidated.
Cephalosporins are a broad class of bactericidal antibiotics that include the β-lactam ring and share a structural similarity and mechanism of action with other β-lactam antibiotics. The cephalosporins have the ability to kill bacteria by inhibiting essential steps in the bacterial cell wall synthesis which in the end results in osmotic lysis and death of the bacterial cell. Cephalosporins are widely used antibiotics because of their clinical efficiency and desirable safety profile.
4-Hydroxyphenylpyruvate dioxygenase (HPPD) inhibitors are a class of herbicides that prevent growth in plants by blocking 4-Hydroxyphenylpyruvate dioxygenase, an enzyme in plants that breaks down the amino acid tyrosine into molecules that are then used by plants to create other molecules that plants need. This process of breakdown, or catabolism, and making new molecules from the results, or biosynthesis, is something all living things do. HPPD inhibitors were first brought to market in 1980, although their mechanism of action was not understood until the late 1990s. They were originally used primarily in Japan in rice production, but since the late 1990s have been used in Europe and North America for corn, soybeans, and cereals, and since the 2000s have become more important as weeds have become resistant to glyphosate and other herbicides. Genetically modified crops are under development that include resistance to HPPD inhibitors. There is a pharmaceutical drug on the market, nitisinone, that was originally under development as an herbicide as a member of this class, and is used to treat an orphan disease, type I tyrosinemia.

Neoxaline is a bio-active Aspergillus japonicus isolate. It is an antimitotic agent and shows weak inhibitory activity of blood platelet aggregation induced by simulation of the central nervous system. It has been synthesized through the "highly stereoselective introduction of a reverse prenyl group to create a quaternary carbon stereocenter using (−)-3a-hydroxyfuroindoline as a building block, construction of the indoline spiroaminal via cautious stepwise oxidations with cyclizations from the indoline, assembly of (Z)-dehydrohistidine, and photoisomerization of unnatural (Z)-neoxaline to the natural (E)-neoxaline."

Bottromycin is a macrocyclic peptide with antibiotic activity. It was first discovered in 1957 as a natural product isolated from Streptomyces bottropensis. It has been shown to inhibit methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE) among other Gram-positive bacteria and mycoplasma. Bottromycin is structurally distinct from both vancomycin, a glycopeptide antibiotic, and methicillin, a beta-lactam antibiotic.
Naphthomycins are a group of closely related antimicrobial chemical compounds isolated from Streptomyces. They are considered a subclass of ansamycins.
Streptomyces isolates have yielded the majority of human, animal, and agricultural antibiotics, as well as a number of fundamental chemotherapy medicines. Streptomyces is the largest antibiotic-producing genus of Actinomycetota, producing chemotherapy, antibacterial, antifungal, antiparasitic drugs, and immunosuppressants. Streptomyces isolates are typically initiated with the aerial hyphal formation from the mycelium.

Satoshi Ōmura is a Japanese biochemist. He is known for the discovery and development of hundreds of pharmaceuticals originally occurring in microorganisms. In 2015, he was awarded the Nobel Prize in Physiology or Medicine jointly with William C. Campbell for their role in the discovery of avermectins and ivermectin, the world's first endectocide and a safe and highly effective microfilaricide. It is believed that the large molecular size of ivermectin prevents it from crossing the blood/aqueous humour barrier, and renders the drug an important treatment of helminthically-derived blindness.
Streptomyces nitrosporeus is a bacterium species from the genus of Streptomyces which has been isolated from garden soil in Japan. Streptomyces nitrosporeus produces Benzastatin E, Benzastatin F, Benzastatin G Nitrosporeusine A and Nitrosporeusine B and the antibiotics nitrosporin and virantomycin and the inhibitor of angiotensin-converting enzyme foroxymithine. Streptomyces nitrosporeus can degrade cellulose.

Bicyclomycin (Bicozamycin) is a broad spectrum antibiotic active against Gram-negative bacteria and the Gram-positive bacterium, Micrococcus luteus that was isolated from Streptomyces sapporonesis and Streptomyces aizumenses in 1972. It belongs to a class of naturally occurring 2,5-diketopiperazines, that are among the most numerous of all the naturally occurring peptide antibiotics. This clinically useful antibiotic is rapidly absorbed in humans when given intramuscularly, has low toxicity and has been used to treat diarrhea in humans and bacterial diarrhea in calves and pigs.
Aspergillus panamensis is a species of fungus in the genus Aspergillus which produces cyclogregatin, gregatin A, gregatin B, gregatin C and gregatin D.

The arylomycins are a class of antibiotics initially isolated from a soil sample obtained in Cape Coast, Ghana. In this initial isolation, two families of closely related arylomycins, A and B, were identified. The family of glycosylated arylomycin C lipopeptides were subsequently isolated from a Streptomyces culture in a screen for inhibitors of bacterial signal peptidase. The initially isolated arylomycins have a limited spectrum of activity against Gram-positive bacteria, including Staphylococcus aureus and Streptococcus pneumoniae. The only activity against Gram-negative bacteria was seen in strains with a compromised outer membrane.

Fluazifop is the ISO common name for an organic compound that is used as a selective herbicide. The active ingredient is the 2R enantiomer at its chiral centre and this material is known as fluazifop-P when used in that form. More commonly, it is sold as its butyl ester, fluazifop-P butyl with the brand name Fusilade.

Halovir refers to a multi-analogue compound belonging to a group of oligopeptides designated as lipopeptaibols which have membrane-modifying capacity and are fungal in origin. These peptides display interesting microheterogeneity; slight variation in encoding amino acids gives rise to a mixture of closely related analogues and have been shown to have antibacterial/antiviral properties.














