
An alloy is a combination of metals or metals combined with one or more other elements. For example, combining the metallic elements gold and copper produces red gold, gold and silver becomes white gold, and silver combined with copper produces sterling silver. Elemental iron, combined with non-metallic carbon or silicon, produces alloys called steel or silicon steel. The resulting mixture forms a substance with properties that often differ from those of the pure metals, such as increased strength or hardness. Unlike other substances that may contain metallic bases but do not behave as metals, such as aluminium oxide (sapphire), beryllium aluminium silicate (emerald) or sodium chloride (salt), an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductility, opaqueness, and luster. Alloys are used in a wide variety of applications, from the steel alloys, used in everything from buildings to automobiles to surgical tools, to exotic titanium-alloys used in the aerospace industry, to beryllium-copper alloys for non-sparking tools. In some cases, a combination of metals may reduce the overall cost of the material while preserving important properties. In other cases, the combination of metals imparts synergistic properties to the constituent metal elements such as corrosion resistance or mechanical strength. Examples of alloys are steel, solder, brass, pewter, duralumin, bronze and amalgams.

Distillation is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation. Distillation may result in essentially complete separation, or it may be a partial separation that increases the concentration of selected components in the mixture. In either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but it is a physical separation process, not a chemical reaction.
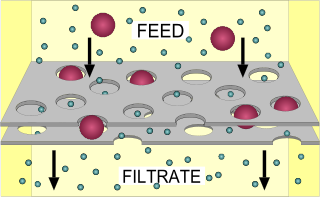
Filtration is a physical, biological or chemical operation that separates solid matter and fluid from a mixture with a filter medium that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. Oversize particles may form a filter cake on top of the filter and may also block the filter lattice, preventing the fluid phase from crossing the filter, known as blinding. The size of the largest particles that can successfully pass through a filter is called the effective pore size of that filter. The separation of solid and fluid is imperfect; solids will be contaminated with some fluid and filtrate will contain fine particles. Filtration occurs both in nature and in engineered systems; there are biological, geological, and industrial forms.

In the physical sciences, a phase is a region of space, throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase.

In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when one of the solvents is water.

Solubility is the property of a solid, liquid or gaseous chemical substance called solute to dissolve in a solid, liquid or gaseous solvent. The solubility of a substance fundamentally depends on the physical and chemical properties of the solute and solvent as well as on temperature, pressure and presence of other chemicals of the solution. The extent of the solubility of a substance in a specific solvent is measured as the saturation concentration, where adding more solute does not increase the concentration of the solution and begins to precipitate the excess amount of solute.

Heat treating is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve the desired result such as hardening or softening of a material. Heat treatment techniques include annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing and quenching. Although the term heat treatment applies only to processes where the heating and cooling are done for the specific purpose of altering properties intentionally, heating and cooling often occur incidentally during other manufacturing processes such as hot forming or welding.

In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions. These can be simple ions such as the sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic species such as the ammonium (NH+
4) and carbonate (CO2−
3) ions in ammonium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbours, so are not considered to be part of molecules, but instead part of a continuous three-dimensional network, usually in a crystalline structure.
In chemistry, a mixture is a material made up of two or more different substances which are physically combined. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the form of solutions, suspensions and colloids.

Precipitation is the process of conversion of a chemical substance into a solid from a solution by converting the substance into an insoluble form or a super-saturated solution. When the reaction occurs in a liquid solution, the solid formed is called the precipitate. The chemical agent that causes the solid to form is called the precipitant.

Industrial processes are procedures involving chemical, physical, electrical or mechanical steps to aid in the manufacturing of an item or items, usually carried out on a very large scale. Industrial processes are the key components of heavy industry.
Impurities are chemical substances inside a confined amount of liquid, gas, or solid, which differ from the chemical composition of the material or compound.
A solid solution describes a family of materials which have a range of compositions e.g. AxB1-x and a single crystal structure. Many examples can be found in metallurgy, geology and solid-state chemistry. The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous materials from physical mixtures of components.

A foundry is a factory that produces metal castings. Metals are cast into shapes by melting them into a liquid, pouring the metal into a mold, and removing the mold material after the metal has solidified as it cools. The most common metals processed are aluminium and cast iron. However, other metals, such as bronze, brass, steel, magnesium, and zinc, are also used to produce castings in foundries. In this process, parts of desired shapes and sizes can be formed.

A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., without breaking chemical bonds. Chemical substances can be simple substances, chemical compounds, or alloys. Chemical elements may or may not be included in the definition, depending on expert viewpoint.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.

Solid is one of the four fundamental states of matter. The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice, or irregularly. Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed.

A chemical compound is a chemical substance composed of many identical molecules composed of atoms from more than one element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound.
A separation process is a method that converts a mixture or solution of chemical substances into two or more distinct product mixtures. At least one of results of the separation is enriched in one or more of the source mixture's constituents. In some cases, a separation may fully divide the mixture into pure constituents. Separations exploit differences in chemical properties or physical properties between the constituents of a mixture.












