
Herbicides, also commonly known as weed killers, are substances used to control undesired plants, also known as weeds. Selective herbicides control specific weed species while leaving the desired crop relatively unharmed, while non-selective herbicides kill plants indiscriminately. The combined effects of herbicides, nitrogen fertilizer, and improved cultivars has increased yields of major crops by 3x to 6x from 1900 to 2000.

Secondary metabolites, also called specialised metabolites, toxins, secondary products, or natural products, are organic compounds produced by any lifeform, e.g. bacteria, fungi, animals, or plants, which are not directly involved in the normal growth, development, or reproduction of the organism. Instead, they generally mediate ecological interactions, which may produce a selective advantage for the organism by increasing its survivability or fecundity. Specific secondary metabolites are often restricted to a narrow set of species within a phylogenetic group. Secondary metabolites often play an important role in plant defense against herbivory and other interspecies defenses. Humans use secondary metabolites as medicines, flavourings, pigments, and recreational drugs.
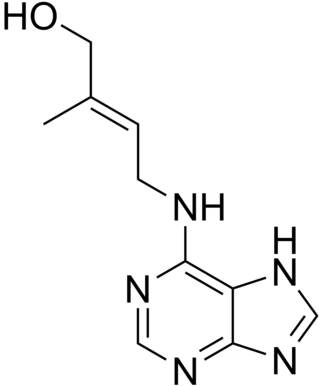
Cytokinins (CK) are a class of plant hormones that promote cell division, or cytokinesis, in plant roots and shoots. They are involved primarily in cell growth and differentiation, but also affect apical dominance, axillary bud growth, and leaf senescence.

In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of Heliconius butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body.

Jasmonate (JA) and its derivatives are lipid-based plant hormones that regulate a wide range of processes in plants, ranging from growth and photosynthesis to reproductive development. In particular, JAs are critical for plant defense against herbivory and plant responses to poor environmental conditions and other kinds of abiotic and biotic challenges. Some JAs can also be released as volatile organic compounds (VOCs) to permit communication between plants in anticipation of mutual dangers.

Ouabain or also known as g-strophanthin, is a plant derived toxic substance that was traditionally used as an arrow poison in eastern Africa for both hunting and warfare. Ouabain is a cardiac glycoside and in lower doses, can be used medically to treat hypotension and some arrhythmias. It acts by inhibiting the Na/K-ATPase, also known as the sodium–potassium ion pump. However, adaptations to the alpha-subunit of the Na+/K+-ATPase via amino acid substitutions, have been observed in certain species, namely some herbivore- insect species, that have resulted in toxin resistance.

Phytochemistry is the study of phytochemicals, which are chemicals derived from plants. Phytochemists strive to describe the structures of the large number of secondary metabolites found in plants, the functions of these compounds in human and plant biology, and the biosynthesis of these compounds. Plants synthesize phytochemicals for many reasons, including to protect themselves against insect attacks and plant diseases. The compounds found in plants are of many kinds, but most can be grouped into four major biosynthetic classes: alkaloids, phenylpropanoids, polyketides, and terpenoids.

A leaf spot is a limited, discoloured, diseased area of a leaf that is caused by fungal, bacterial or viral plant diseases, or by injuries from nematodes, insects, environmental factors, toxicity or herbicides. These discoloured spots or lesions often have a centre of necrosis. Symptoms can overlap across causal agents, however differing signs and symptoms of certain pathogens can lead to the diagnosis of the type of leaf spot disease. Prolonged wet and humid conditions promote leaf spot disease and most pathogens are spread by wind, splashing rain or irrigation that carry the disease to other leaves.

Lupinine is a quinolizidine alkaloid present in the genus Lupinus of the flowering plant family Fabaceae. The scientific literature contains many reports on the isolation and synthesis of this compound as well as a vast number of studies on its biosynthesis from its natural precursor, lysine. Studies have shown that lupinine hydrochloride is a mildly toxic acetylcholinesterase inhibitor and that lupinine has an inhibitory effect on acetylcholine receptors. The characteristically bitter taste of lupin beans, which come from the seeds of Lupinus plants, is attributable to the quinolizidine alkaloids which they contain, rendering them unsuitable for human and animal consumption unless handled properly. However, because lupin beans have potential nutritional value due to their high protein content, efforts have been made to reduce their alkaloid content through the development of "sweet" varieties of Lupinus.

Pseudomonas syringae is a rod-shaped, Gram-negative bacterium with polar flagella. As a plant pathogen, it can infect a wide range of species, and exists as over 50 different pathovars, all of which are available to researchers from international culture collections such as the NCPPB, ICMP, and others.

Pseudomonas savastanoi is a gram-negative plant pathogenic bacterium that infects a variety of plants. It was once considered a pathovar of Pseudomonas syringae, but following DNA-relatedness studies, it was instated as a new species. It is named after Savastano, a worker who proved between 1887 and 1898 that olive knot are caused by bacteria.

Pseudomonas cannabina is a gray, Gram-negative, fluorescent, motile, flagellated, aerobic bacterium that causes leaf and stem rot of hemp, from which it derives its name. It was formerly classified as a pathovar of Pseudomonas syringae, but following ribotypical analysis, it was reinstated as a species. The type strain is CFBP 2341.

Halo blight of bean is a bacterial disease caused by Pseudomonas syringae pv. phaseolicola. Halo blight’s pathogen is a gram-negative, aerobic, polar-flagellated and non-spore forming bacteria. This bacterial disease was first discovered in the early 1920s, and rapidly became the major disease of beans throughout the world. The disease favors the places where temperatures are moderate and plentiful inoculum is available.

Betaenone B, like other betaenones, is a secondary metabolite isolated from the fungus Pleospora betae, a plant pathogen. Its phytotoxic properties have been shown to cause sugar beet leaf spots, which is characterized by black, pycnidia containing, concentric circles eventually leading to necrosis of the leaf tissue. Of the seven phytotoxins isolated in fungal leaf spots from sugar beet, betaenone B showed the least amount of phytotoxicity showing only 8% inhibition of growth while betaenone A and C showed 73% and 89% growth inhibition, respectively. Betaenone B is therefore not considered toxic to the plant, but will produce leaf spots when present in high concentrations (0.33 μg/μL). While the mechanism of action of betaenone B has yet to be elucidated, betaenone C has been shown to inhibit RNA and protein synthesis. Most of the major work on betaenone B, including the initial structure elucidation of betaenone A, B and C as well as the partial elucidation mechanism of biosynthesis, was presented in three short papers published between 1983 and 1988. The compounds were found to inhibit a variety of protein kinases signifying a possible role in cancer treatment.

Tabtoxin, also known as wildfire toxin, is a simple monobactam phytotoxin produced by Pseudomonas syringae. It is the precursor to the antibiotic tabtoxinine β-lactam (TBL). It is produced by:

Angelicin is the parent compound in a family of naturally occurring organic compounds known as the angular furanocoumarins. Structurally, it can be considered as benzapyra-2-one fused with a furan moiety in the 7,8-position. Angelicin is commonly found in certain Apiaceae and Fabaceae plant species such as Bituminaria bituminosa. It has a skin permeability coefficient (LogKp) of -2.46. The maximum absorption is observed at 300 nm. The 1HNMR spectrum is available; the infrared and mass spectra of angelicin can be found in this database. The sublimation of angelicin occurs at 120 °C and the pressure of 0.13 Pa. Angelicin is a coumarin.

Secondary metabolism produces a large number of specialized compounds that do not aid in the growth and development of plants but are required for the plant to survive in its environment. Secondary metabolism is connected to primary metabolism by using building blocks and biosynthetic enzymes derived from primary metabolism. Primary metabolism governs all basic physiological processes that allow a plant to grow and set seeds, by translating the genetic code into proteins, carbohydrates, and amino acids. Specialized compounds from secondary metabolism are essential for communicating with other organisms in mutualistic or antagonistic interactions. They further assist in coping with abiotic stress such as increased UV-radiation. The broad functional spectrum of specialized metabolism is still not fully understood. In any case, a good balance between products of primary and secondary metabolism is best for a plant’s optimal growth and development as well as for its effective coping with often changing environmental conditions. Well known specialized compounds include alkaloids, polyphenols including flavonoids, and terpenoids. Humans use many of these compounds for culinary, medicinal and nutraceutical purposes.
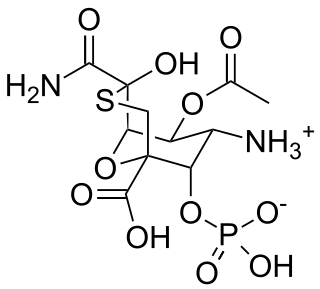
Tagetitoxin (TGT) is a bacterial phytotoxin produced by Pseudomonas syringae pv. tagetis.

Bacterial blight of soybean is a widespread disease of soybeans caused by Pseudomonas syringaepv. glycinea.


















