Related Research Articles
In chemistry, an alkali is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases.

In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells.

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.

Dietary fiber or roughage is the portion of plant-derived food that cannot be completely broken down by human digestive enzymes. Dietary fibers are diverse in chemical composition and can be grouped generally by their solubility, viscosity and fermentability which affect how fibers are processed in the body. Dietary fiber has two main components: soluble fiber and insoluble fiber which are components of plant-based foods such as legumes, whole grains, cereals, vegetables, fruits, and nuts or seeds. A diet high in regular fiber consumption is generally associated with supporting health and lowering the risk of several diseases. Dietary fiber consists of non-starch polysaccharides and other plant components such as cellulose, resistant starch, resistant dextrins, inulin, lignins, chitins, pectins, beta-glucans, and oligosaccharides.

In chemistry, there are three definitions in common use of the word "base": Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century.
Rancidification is the process of complete or incomplete autoxidation or hydrolysis of fats and oils when exposed to air, light, moisture, or bacterial action, producing short-chain aldehydes, ketones and free fatty acids.
Saponification is a process of cleaving esters into carboxylate salts and alcohols by the action of aqueous alkali. Typically aqueous sodium hydroxide solutions are used. It is an important type of alkaline hydrolysis. When the carboxylate is long chain, its salt is called a soap. The saponification of ethyl acetate gives sodium acetate and ethanol:

Palmitic acid is a fatty acid with a 16-carbon chain. It is the most common saturated fatty acid found in animals, plants and microorganisms. Its chemical formula is CH3(CH2)14COOH, and its C:D ratio is 16:0. It is a major component of palm oil from the fruit of Elaeis guineensis, making up to 44% of total fats. Meats, cheeses, butter, and other dairy products also contain palmitic acid, amounting to 50–60% of total fats.

Saponification value or saponification number represents the number of milligrams of potassium hydroxide (KOH) or sodium hydroxide (NaOH) required to saponify one gram of fat under the conditions specified. It is a measure of the average molecular weight of all the fatty acids present in the sample in form of triglycerides. The higher the saponification value, the lower the fatty acids average length, the lighter the mean molecular weight of triglycerides and vice versa. Practically, fats or oils with high saponification value are more suitable for soap making.
Fatty acid methyl esters (FAME) are a type of fatty acid ester that are derived by transesterification of fats with methanol. The molecules in biodiesel are primarily FAME, usually obtained from vegetable oils by transesterification. They are used to produce detergents and biodiesel. FAME are typically produced by an alkali-catalyzed reaction between fats and methanol in the presence of base such as sodium hydroxide, sodium methoxide or potassium hydroxide. One reason for using FAME in biodiesel production, rather than free fatty acids, is to mitigate the potential corrosion they can cause to metals of engines, production facilities, and related infrastructure. While free fatty acids are only mildly acidic, over time they can lead to cumulative corrosion. In contrast, their esters, such as FAME, are less corrosive and therefore preferred for biodiesel production. As an improved quality, FAMEs also usually have about 12-15 units higher cetane number than their unesterified counterparts.
In chemistry, acid value is a number used to quantify the acidity of a given chemical substance. It is the quantity of base, expressed as milligrams of KOH required to neutralize the acidic constituents in 1 gram of a sample. The acid value measures the acidity of water-insoluble substances like oils, fats, waxes and resins, which do not have a pH value.
Resin acid refers to mixtures of several related carboxylic acids, primarily abietic acid, found in tree resins. Nearly all resin acids have the same basic skeleton: three fused rings having the empirical formula C19H29COOH. Resin acids are tacky, yellowish gums that are water-insoluble. They are used to produce soaps for diverse applications, but their use is being displaced increasingly by synthetic acids such as 2-ethylhexanoic acid or petroleum-derived naphthenic acids.
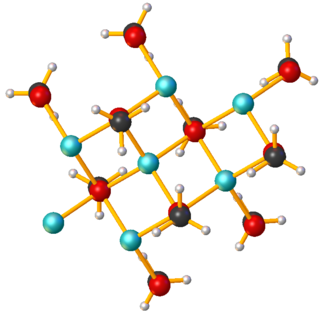
Sodium methoxide is the simplest sodium alkoxide. With the formula CH3ONa, it is a white solid, which is formed by the deprotonation of methanol. It is a widely used reagent in industry and the laboratory. It is also a dangerously caustic base.
The Reichert value is a value determined when examining fats and oils. The Reichert value is an indicator of how much volatile fatty acid can be extracted from a particular fat or oil through saponification. It is equal to the number of millilitres of 0.1 normal hydroxide solution necessary for the neutralization of the water-soluble volatile fatty acids distilled and filtered from 5 grams of a given saponified fat.
The Kirschner value or Kirschner number is a value determined when examining fat. The Kirschner value is an indicator of how much volatile fatty acid can be extracted from fat through saponification. It consists of the number of milliliters of 0.1 normal sodium hydroxide necessary for the neutralization of water-soluble silver salts made from the water-soluble volatile fatty acids distilled from 5 grams of a given fat.
In chemistry, the terms volatile acid and volatile acidity (VA) are used somewhat differently in various application areas.
In analytical chemistry, the hydroxyl value is defined as the number of milligrams of potassium hydroxide (KOH) required to neutralize the acetic acid taken up on acetylation of one gram of a chemical substance that contains free hydroxyl groups. The analytical method used to determine hydroxyl value traditionally involves acetylation of the free hydroxyl groups of the substance with acetic anhydride in pyridine solvent. After completion of the reaction, water is added, and the remaining unreacted acetic anhydride is converted to acetic acid and measured by titration with potassium hydroxide.

Hard soaps, also termed soda soaps in older terminology, are categorized under soaps and are typically sodium salts of fatty acids. They vary in color from white to brownish and have a fatty acid content ranging from 72 to 75%. These soaps are typically made from lower-quality fats. Hard soaps serve as the foundation for products frequently labeled as fine soaps, which are fortified with nourishing additives, perfumes, and dyes.
Saltwater soap, also called sailors' soap, is a potassium-based soap for use with seawater. Inexpensive common commercial soap will not lather or dissolve in seawater due to high levels of sodium chloride in the water. Similarly, common soap does not work as well as potassium-based soap in hard water where calcium replaces the sodium, making residual insoluble "scum" due to the insolubility of the soap residue. To be an effective cleaning agent, soap must be able to dissolve in water.
References
- ↑ Food and Agriculture Organization and World Health Organization. (2001). The Codex Alimentarius . Rome:FAO/WHO. Volume 8: Fats and Oils, "Section 4.9.1: Estimation of Milk Fat Content". ISBN 92-5-104682-4
- ↑ "Polenske value", Merriam-Webster Medical Dictionary (online).