
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose, and alginate. Other examples of biopolymers include natural rubbers, suberin and lignin, cutin and cutan, melanin, and polyhydroxyalkanoates (PHAs).

A polymer is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.
A polyamide is a polymer with repeating units linked by amide bonds.

In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called bipolymers. Those obtained from three and four monomers are called terpolymers and quaterpolymers, respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material.

Polyglycolide or poly(glycolic acid) (PGA), also spelled as polyglycolic acid, is a biodegradable, thermoplastic polymer and the simplest linear, aliphatic polyester. It can be prepared starting from glycolic acid by means of polycondensation or ring-opening polymerization. PGA has been known since 1954 as a tough fiber-forming polymer. Owing to its hydrolytic instability, however, its use has initially been limited. Currently polyglycolide and its copolymers (poly(lactic-co-glycolic acid) with lactic acid, poly(glycolide-co-caprolactone) with ε-caprolactone and poly (glycolide-co-trimethylene carbonate) with trimethylene carbonate) are widely used as a material for the synthesis of absorbable sutures and are being evaluated in the biomedical field.
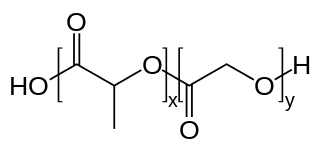
PLGA, PLG, or poly(lactic-co-glycolic) acid is a copolymer which is used in a host of Food and Drug Administration (FDA) approved therapeutic devices, owing to its biodegradability and biocompatibility. PLGA is synthesized by means of ring-opening co-polymerization of two different monomers, the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. Polymers can be synthesized as either random or block copolymers thereby imparting additional polymer properties. Common catalysts used in the preparation of this polymer include tin(II) 2-ethylhexanoate, tin(II) alkoxides, or aluminum isopropoxide. During polymerization, successive monomeric units are linked together in PLGA by ester linkages, thus yielding a linear, aliphatic polyester as a product.
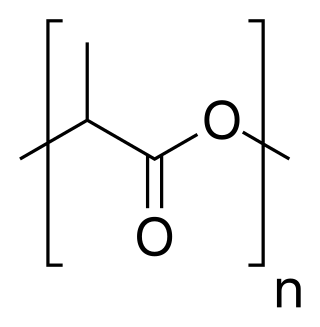
Polylactic acid, also known as poly(lactic acid) or polylactide (PLA), is a thermoplastic polyester with backbone formula (C
3H
4O
2)
n or [–C(CH
3)HC(=O)O–]
n, formally obtained by condensation of lactic acid C(CH
3)(OH)HCOOH with loss of water. It can also be prepared by ring-opening polymerization of lactide [–C(CH
3)HC(=O)O–]
2, the cyclic dimer of the basic repeating unit.

Hot-melt adhesive (HMA), also known as hot glue, is a form of thermoplastic adhesive that is commonly sold as solid cylindrical sticks of various diameters designed to be applied using a hot glue gun. The gun uses a continuous-duty heating element to melt the plastic glue, which the user pushes through the gun either with a mechanical trigger mechanism on the gun, or with direct finger pressure. The glue squeezed out of the heated nozzle is initially hot enough to burn and even blister skin. The glue is sticky when hot, and solidifies in a few seconds to one minute. Hot-melt adhesives can also be applied by dipping or spraying, and are popular with hobbyists and crafters both for affixing and as an inexpensive alternative to resin casting.

Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
Biodegradable polymers are a special class of polymer that breaks down after its intended purpose by bacterial decomposition process to result in natural byproducts such as gases (CO2, N2), water, biomass, and inorganic salts. These polymers are found both naturally and synthetically made, and largely consist of ester, amide, and ether functional groups. Their properties and breakdown mechanism are determined by their exact structure. These polymers are often synthesized by condensation reactions, ring opening polymerization, and metal catalysts. There are vast examples and applications of biodegradable polymers.
Many opportunities exist for the application of synthetic biodegradable polymers in the biomedical area particularly in the fields of tissue engineering and controlled drug delivery. Degradation is important in biomedicine for many reasons. Degradation of the polymeric implant means surgical intervention may not be required in order to remove the implant at the end of its functional life, eliminating the need for a second surgery. In tissue engineering, biodegradable polymers can be designed such to approximate tissues, providing a polymer scaffold that can withstand mechanical stresses, provide a suitable surface for cell attachment and growth, and degrade at a rate that allows the load to be transferred to the new tissue. In the field of controlled drug delivery, biodegradable polymers offer tremendous potential either as a drug delivery system alone or in conjunction to functioning as a medical device.

Poly(3-hydroxybutyrate-co-3-hydroxyvalerate), commonly known as PHBV, is a polyhydroxyalkanoate-type polymer. It is biodegradable, nontoxic, biocompatible plastic produced naturally by bacteria and a good alternative for many non-biodegradable synthetic polymers. It is a thermoplastic linear aliphatic polyester. It is obtained by the copolymerization of 3-hydroxybutanoic acid and 3-hydroxypentanoic acid. PHBV is used in speciality packaging, orthopedic devices and in controlled release of drugs. PHBV undergoes bacterial degradation in the environment.
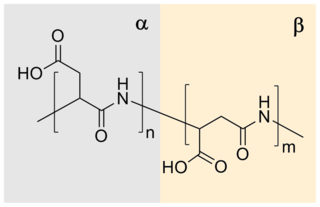
Polyaspartic acid (PASA) is a biodegradable, water-soluble condensation polymer based on the amino acid aspartic acid. It is a biodegradable replacement for water softeners and related applications. PASA can be chemically crosslinked with a wide variety of methods to yield PASA hydrogels. The resulting hydrogels are pH-sensitive such that under acidic conditions, they shrink, while the swelling capacity increases under alkaline conditions.
Poly(ethylene adipate) or PEA is an aliphatic polyester. It is most commonly synthesized from a polycondensation reaction between ethylene glycol and adipic acid. PEA has been studied as it is biodegradable through a variety of mechanisms and also fairly inexpensive compared to other polymers. Its lower molecular weight compared to many polymers aids in its biodegradability.

Biodegradable athletic footwear is athletic footwear that uses biodegradable materials with the ability to compost at the end-of-life phase. Such materials include natural biodegradable polymers, synthetic biodegradable polymers, and biodegradable blends. The use of biodegradable materials is a long-term solution to landfill pollution that can significantly help protect the natural environment by replacing the synthetic, non-biodegradable polymers found in athletic footwear.
Polyorthoesters are polymers with the general structure –[–R–O–C(R1, OR2)–O–R3–]n– whereas the residue R2 can also be part of a heterocyclic ring with the residue R. Polyorthoesters are formed by transesterification of orthoesters with diols or by polyaddition between a diol and a diketene acetal, such as 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane.
3,9-Diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane (DETOSU) is a bicyclic ketene acetal derived from the isomeric allyl acetal 3,9-divinyl-2,4,8,10-tetraoxaspiro[5.5]undecane (DVTOSU). As a bifunctional monomer, DETOSU is an important building block for polyorthoesters formed by the addition of diols to the activated double bond of the diketene acetal.
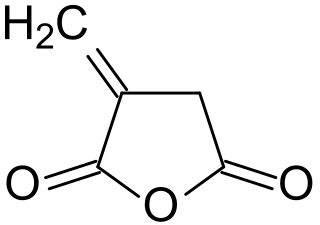
Itaconic anhydride is the cyclic anhydride of itaconic acid and is obtained by the pyrolysis of citric acid. It is a colourless, crystalline solid, which dissolves in many polar organic solvents and hydrolyzes forming itaconic acid. Itaconic anhydride and its derivative itaconic acid have been promoted as biobased "platform chemicals" and bio- building blocks.) These expectations, however, have not been fulfilled.
The methods for sequence analysis of synthetic polymers differ from the sequence analysis of biopolymers. Synthetic polymers are produced by chain-growth or step-growth polymerization and show thereby polydispersity, whereas biopolymers are synthesized by complex template-based mechanisms and are sequence-defined and monodisperse. Synthetic polymers are a mixture of macromolecules of different length and sequence and are analysed via statistical measures.

Poly(trimethylene carbonate) (PTMC) is an aliphatic polycarbonate synthesized from the 6-membered cyclic carbonate, trimethylene carbonate (1,3-propylene carbonate or 1,3-Dioxan-2-one). Trimethylene carbonate (TMC) is a colorless crystalline solid with melting point ranging between 45°C and 48 °C and boiling point at 255°C (at 760 mmHg). TMC is originally synthesized from 1,3-propanediol with phosgene or carbon monoxide, which are highly poisonous gases. Another route is from the transesterification of 1,3-propanediol and dialkylcarbonates. This route is considered "greener" compared to the other one, since precursors can be obtained from renewable resources and carbon dioxide.














