Related Research Articles

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Ethylene glycol is an organic compound with the formula (CH2OH)2. It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. It has a sweet taste, but is toxic in high concentrations. This molecule has been observed in outer space.

Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular weight. The structure of PEG is commonly expressed as H−(O−CH2−CH2)n−OH.

Laxatives, purgatives, or aperients are substances that loosen stools and increase bowel movements. They are used to treat and prevent constipation.
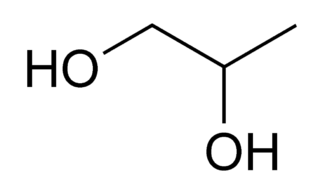
Propylene glycol (IUPAC name: propane-1,2-diol) is a viscous, colorless liquid, which is nearly odorless but possesses a faintly sweet taste. Its chemical formula is CH3CH(OH)CH2OH. As it contains two alcohol groups, it is classed as a diol. It is miscible with a broad range of solvents, including water, acetone, and chloroform. In general, glycols are non-irritating and have very low volatility.

Stearic acid is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a soft waxy solid with the formula CH3(CH2)16CO2H. The triglyceride derived from three molecules of stearic acid is called stearin. Stearic acid is a prevalent fatty-acid in nature, found in many animal and vegetable fats, but is usually higher in animal fat than vegetable fat. It has a melting point of 69.4 °C and a pKa of 4.50.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
An antifreeze is an additive which lowers the freezing point of a water-based liquid. An antifreeze mixture is used to achieve freezing-point depression for cold environments. Common antifreezes also increase the boiling point of the liquid, allowing higher coolant temperature. However, all common antifreeze additives also have lower heat capacities than water, and do reduce water's ability to act as a coolant when added to it.
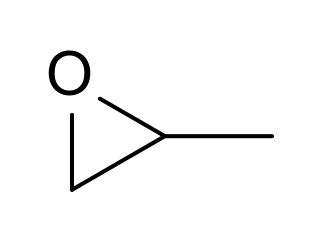
Propylene oxide is an acutely toxic and carcinogenic organic compound with the molecular formula C3H6O. This colourless volatile liquid with an odour similar to ether, is produced on a large scale industrially. Its major application is its use for the production of polyether polyols for use in making polyurethane plastics. It is a chiral epoxide, although it is commonly used as a racemic mixture.
A humectant is a hygroscopic (water-absorbing) substance used to keep things moist. They are used in many products, including food, cosmetics, medicines and pesticides. When used as a food additive, a humectant has the effect of keeping moisture in the food. Humectants are sometimes used as a component of antistatic coatings for plastics.
Excipient is a substance formulated alongside the active ingredient of a medication. Excipients serve various purposes, including long-term stabilization, bulking up solid formulations containing potent active ingredients in small amounts, or enhancing the therapeutic properties of the active ingredient in the final dosage form. They can facilitate drug absorption, reduce viscosity, or enhance solubility. Excipients can also aid in the manufacturing process by improving the handling of active substances, facilitating powder flowability, or preventing denaturation and aggregation during the expected shelf life. The selection of excipients depends on factors such as the route of administration, dosage form, and active ingredient.

Glycol stearate (glycol monostearate or ethylene glycol monostearate) is an organic compound with the molecular formula C20H40O3. It is the ester of stearic acid and ethylene glycol. It is used as an ingredient in many types of personal care products and cosmetics including shampoos, hair conditioners, and skin lotions.
In organic chemistry, ethoxylation is a chemical reaction in which ethylene oxide adds to a substrate. It is the most widely practiced alkoxylation, which involves the addition of epoxides to substrates.
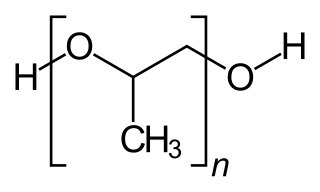
Polypropylene glycol or polypropylene oxide is the polymer of propylene glycol. Chemically it is a polyether, and, more generally speaking, it's a polyalkylene glycol (PAG) H S Code 3907.2000. The term polypropylene glycol or PPG is reserved for polymer of low- to medium-range molar mass when the nature of the end-group, which is usually a hydroxyl group, still matters. The term "oxide" is used for high-molar-mass polymer when end-groups no longer affect polymer properties. Between 60 and 70% of propylene oxide is converted to polyether polyols by the process called alkoxylation.
Glycol ethers are a class of chemical compounds consisting of alkyl ethers that are based on glycols such as ethylene glycol or propylene glycol. They are commonly used as solvents in paints and cleaners. They have good solvent properties while having higher boiling points than the lower-molecular-weight ethers and alcohols.
Poloxamer 407 is a hydrophilic non-ionic surfactant of the more general class of copolymers known as poloxamers. Poloxamer 407 is a triblock copolymer consisting of a central hydrophobic block of polypropylene glycol flanked by two hydrophilic blocks of polyethylene glycol (PEG). The approximate lengths of the two PEG blocks is 101 repeat units, while the approximate length of the propylene glycol block is 56 repeat units. This particular compound is also known by the BASF trade name Pluronic F-127 or by the Croda trade name Synperonic PE/F 127. BASF also offers a pharmaceutical grade, under trade name Kolliphor P 407.
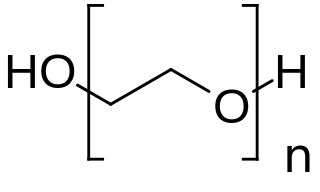
Macrogol, also known as polyethylene glycol (PEG), is used as a medication to treat constipation in children and adults. It is taken by mouth. Benefits usually occur within three days. Generally it is only recommended for up to two weeks. It is also used as an excipient. It is also used to clear the bowels before a colonoscopy, when the onset of the laxative effect is more rapid, typically within an hour.
Esoterica is an over-the-counter topical ointment applied to the skin for the purpose of lightening freckles, age spots, chloasma, melasma, and other skin discolorations due to a benign localized increase in the production of melanin. Esoterica may have other appropriate medical uses as determined by a physician.

Glycol distearate is the diester of stearic acid and ethylene glycol. It is mostly commonly encountered in personal care products and cosmetics where it is used to produce pearlescent effects as well as a moisturizer.

Sucrose esters or sucrose fatty acid esters are a group of non-naturally occurring surfactants chemically synthesized from the esterification of sucrose and fatty acids. This group of substances is remarkable for the wide range of hydrophilic-lipophilic balance (HLB) that it covers. The polar sucrose moiety serves as a hydrophilic end of the molecule, while the long fatty acid chain serves as a lipophilic end of the molecule. Due to this amphipathic property, sucrose esters act as emulsifiers; i.e., they have the ability to bind both water and oil simultaneously. Depending on the HLB value, some can be used as water-in-oil emulsifiers, and some as oil-in-water emulsifiers. Sucrose esters are used in cosmetics, food preservatives, food additives, and other products. A class of sucrose esters with highly substituted hydroxyl groups, olestra, is also used as a fat replacer in food.
References
- ↑ Johnson Jr, W.; Cosmetic Ingredient Review Expert Panel (4 January 2001). "Final Report on the Safety Assessment of PEG-25 Propylene Glycol Stearate, PEG-75 Propylene Glycol Stearate, PEG-120 Propylene Glycol Stearate, PEG-10 Propylene Glycol, PEG-8 Propylene Glycol Cocoate, and PEG-55 Propylene Glycol Oleate". International Journal of Toxicology. Taylor and Francis Ltd. 20, Supplement 4 (6): 13–26. doi: 10.1080/10915810152902556 . PMID 11800049. S2CID 208149143.
- ↑ "2006/257/EC: Commission Decision of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products (Text with EEA relevance)". Publications Office of the European Union. 2006. Retrieved 2010-10-30..