A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes (alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility:

Polyethylene or polythene is the most common plastic in use today. It is a polymer, primarily used for packaging. As of 2017, over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market.
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar. Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups.
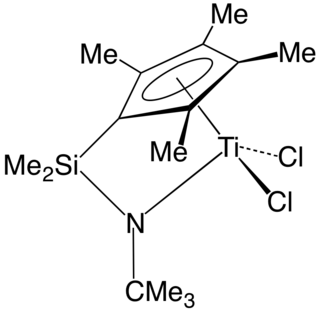
A Kaminsky catalyst is a catalytic system for alkene polymerization. Kaminsky catalysts are based on metallocenes of group 4 transition metals activated with methylaluminoxane (MAO). These and other innovations have inspired development of new classes of catalysts that in turn led to commercialization of novel engineering polyolefins.
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid-gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts.
Polyketones are a family of high-performance thermoplastic polymers. The polar ketone groups in the polymer backbone of these materials gives rise to a strong attraction between polymer chains, which increases the material's melting point (255 °C for copolymer, 220 °C for terpolymer. Trade names include Poketone, Carilon, Karilon, Akrotek, and Schulaketon. Such materials also tend to resist solvents and have good mechanical properties. Unlike many other engineering plastics, aliphatic polyketones such as Shell Chemicals' Carilon are relatively easy to synthesize and can be derived from inexpensive monomers. Carilon is made with a palladium catalyst from ethylene and carbon monoxide. A small fraction of the ethylene is generally replaced with propylene to reduce the melting point somewhat. Shell Chemical commercially launched Carilon thermoplastic polymer in the U.S. in 1996, but discontinued it in 2000. SRI International offers Carilon thermoplastic polymers. Hyosung announced that they would launch production in 2015.

Linear low-density polyethylene (LLDPE) is a substantially linear polymer (polyethylene), with significant numbers of short branches, commonly made by copolymerization of ethylene with longer-chain olefins. Linear low-density polyethylene differs structurally from conventional low-density polyethylene (LDPE) because of the absence of long chain branching and impurity. The linearity of LLDPE results is almost same as LDPE because LDPE is recycled to make LLDPE. In general, LLDPE is produced at lower temperatures and pressures by copolymerization of ethylene and such higher alpha-olefins as butene, hexene, or octene. The copolymerization process produces an LLDPE polymer that has a narrower molecular weight distribution than conventional LDPE and in combination with the linear structure, significantly different rheological properties.
Coordination polymerisation is a form of polymerization that is catalyzed by transition metal salts and complexes.
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More specialized polyolefins include polyisobutylene and polymethylpentene. They are all colorless or white oils or solids. Many copolymers are known, such as polybutene, which derives from a mixture of different butene isomers. The name of each polyolefin indicates the olefin from which it is prepared; for example, polyethylene is derived from ethylene, and polymethylpentene is derived from 4-methyl-1-pentene. Polyolefins are not olefins themselves because the double bond of each olefin monomer is opened in order to form the polymer. Monomers having more than one double bond such as butadiene and isoprene yield polymers that contain double bonds (polybutadiene and polyisoprene) and are usually not considered polyolefins. Polyolefins are the foundations of many chemical industries.

In organometallic chemistry, a "constrained geometry complex" (CGC) is a kind of catalyst used for the production of polyolefins such as polyethylene and polypropylene. The catalyst was one of the first major departures from metallocene-based catalysts and ushered in much innovation in the development of new plastics.

Steven Dale Ittel is an American chemist specializing in organometallic chemistry and homogeneous catalysis.
In 1957, the research organization of the Chemicals Department of E. I. du Pont de Nemours and Company was renamed Central Research Department, beginning the history of the premier scientific organization within DuPont and one of the foremost industrial laboratories devoted to basic science. Located primarily at the DuPont Experimental Station and Chestnut Run, in Wilmington, Delaware, it has expanded to include laboratories in Geneva, Switzerland, Seoul, South Korea, Shanghai, China, and India(Hyderabad). In January, 2016 a major layoff marked the end of the organization.

Maurice S. Brookhart is an American chemist, and professor of chemistry at the University of Houston since 2015.

John E. Bercaw is an American chemist and Centennial Professor of Chemistry, Emeritus at the California Institute of Technology.
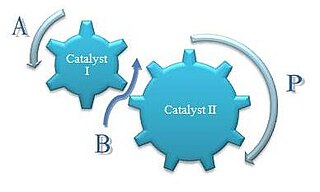
Concurrent tandem catalysis (CTC) is a technique in chemistry where multiple catalysts produce a product otherwise not accessible by a single catalyst. It is usually practiced as homogeneous catalysis. Scheme 1 illustrates this process. Molecule A enters this catalytic system to produce the comonomer, B, which along with A enters the next catalytic process to produce the final product, P. This one-pot approach can decrease product loss from isolation or purification of intermediates. Reactions with relatively unstable products can be generated as intermediates because they are only transient species and are immediately used in a consecutive reaction.
In polymer chemistry, chain walking (CW) or chain running or chain migration is a mechanism that operates during some alkene polymerization reactions. CW can be also considered as a specific case of intermolecular chain transfer. This reaction gives rise to branched and hyperbranched/dendritic hydrocarbon polymers. This process is also characterized by accurate control of polymer architecture and topology. The extent of CW, displayed in the number of branches formed and positions of branches on the polymers are controlled by the choice of a catalyst. The potential applications of polymers formed by this reaction are diverse, from drug delivery to phase transfer agents, nanomaterials, and catalysis.

Brookhart's acid is the salt of the diethyl ether oxonium ion and tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (BAr′4). It is a colorless solid, used as a strong acid. The compound was first reported by Volpe, Grant, and Brookhart in 1992.
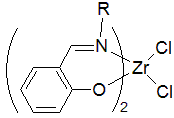
Diiminopyridines are a class of diimine ligands. They featuring a pyridine nucleus with imine sidearms appended to the 2,6–positions. The three nitrogen centres bind metals in a tridentate fashion, forming pincer complexes. Diiminopyridines are notable as non-innocent ligand that can assume more than one oxidation state. Complexes of DIPs participate in a range of chemical reactions, including ethylene polymerization, hydrosilylation, and hydrogenation.
Diimines are organic compounds containing two imine (RCH=NR') groups. Common derivatives are 1,2-diketones and 1,3-diimines. These compounds are used as ligands and as precursors to heterocycles. Diimines are prepared by condensation reactions where a dialdehyde or diketone is treated with amine and water is eliminated. Similar methods are used to prepare Schiff bases and oximes.
Functionalized polyolefins are olefin polymers with polar and nonpolar functionalities attached onto the polymer backbone. There has been an increased interest in functionalizing polyolefins due to their increased usage in everyday life. Polyolefins are virtually ubiquitous in everyday life, from consumer food packaging to biomedical applications; therefore, efforts must be made to study catalytic pathways towards the attachment of various functional groups onto polyolefins in order to affect the material's physical properties.