A post-metallocene catalyst is a kind of catalyst for the polymerization of olefins, i.e., the industrial production of some of the most common plastics. "Post-metallocene" refers to a class of homogeneous catalysts that are not metallocenes. This area has attracted much attention because the market for polyethylene, polypropylene, and related copolymers is large. There is a corresponding intense market for new processes as indicated by the fact that, in the US alone, 50,000 patents were issued between 1991-2007 on polyethylene and polypropylene.
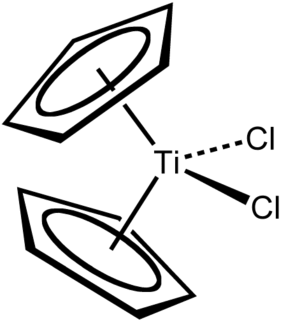
Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.
Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds. Ordinarily the reaction is conducted catalytically and usually the substrates are unsaturated organic compounds. Alkenes and alkynes give alkyl and vinyl silanes; aldehydes and ketones give silyl ethers. Hydrosilylation has been called the "most important application of platinum in homogeneous catalysis."

George W. Parshall was an American organometallic chemist who made notable contributions to homogeneous catalysis. He was a senior scientist at E. I. du Pont de Nemours and Company for many years.
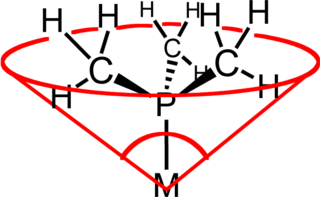
In coordination chemistry, the ligand cone angle is a measure of the steric bulk of a ligand in a transition metal coordination complex. It is defined as the solid angle formed with the metal at the vertex and the outermost edge of the van der Waals spheres of the ligand atoms at the perimeter of the cone. Tertiary phosphine ligands are commonly classified using this parameter, but the method can be applied to any ligand. The term cone angle was first introduced by Chadwick A. Tolman, a research chemist at DuPont. Tolman originally developed the method for phosphine ligands in nickel complexes, determining them from measurements of accurate physical models.
In 1957, the research organization of the Chemicals Department of E. I. du Pont de Nemours and Company was renamed Central Research Department, beginning the history of the premier scientific organization within DuPont and one of the foremost industrial laboratories devoted to basic science. Located primarily at the DuPont Experimental Station and Chestnut Run, in Wilmington, Delaware, it has expanded to include laboratories in Geneva, Switzerland, Seoul, South Korea, Shanghai, China, and India(Hyderabad). In January, 2016 a major layoff marked the end of the organization.

Jean-Marie Basset is a French chemist, and is currently the director of KAUST catalysis research center.
Martin Arthur Bennett FRS is an Australian inorganic chemist. He gained recognition for studies on the co-ordination chemistry of tertiary phosphines, olefins, and acetylenes, and the relationship of their behaviour to homogeneous catalysis.

Organorhodium chemistry is the chemistry of organometallic compounds containing a rhodium-carbon chemical bond, and the study of rhodium and rhodium compounds as catalysts in organic reactions.

Iron tetracarbonyl dihydride is the organometallic compound with the formula H2Fe(CO)4. This compound was the first transition metal hydride discovered. The complex is stable at low temperatures but decomposes rapidly at temperatures above –20 °C.
Catalytic chain transfer (CCT) is a process that can be incorporated into radical polymerization to obtain greater control over the resulting products.

Earl Muetterties, was an American inorganic chemist born in Illinois, who is known for his experimental work with boranes, homogeneous catalysis, heterogeneous catalysis, fluxional processes in organometallic complexes and apicophilicity.

Chadwick A. Tolman is an American chemist. He obtained his B.S. in Chemistry from Massachusetts Institute of Technology. He earned his Ph.D. in Chemistry as a microwave spectroscopist from U.C. Berkeley under the guidance of William Dulaney Gwinn.
Tolman's rule states that, in a certain chemical reaction, the steps involve exclusively intermediates of 18- and 16 electron configuration. The rule is an extension of the 18-electron rule. This rule was proposed by American chemist Chadwick A. Tolman. As stated above, Tolman's rule, even for reactions that proceed via 2e− steps, is incorrect because many reactions involve configurations of fewer than 16 e−.

A metal-phosphine complex is a In coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).

Frederick Nye Tebbe was a chemist known for his work on organometallic chemistry. Tebbe was born in Oakland, California on March 20, 1935. His father, Charles L. Tebbe, worked for the United States Forest Service so Fred’s early education took place in Montana, Oregon, Maryland and Pennsylvania. He married Margaret Manzer in 1960, and they had a son and a daughter. He died of pancreatic cancer at his home in Delaware on September 28, 1995.
William Charles Drinkard, Jr. was an American industrial chemist and the inventor of the catalytic hydrocyanation process for making adiponitrile, a key intermediate in nylon production.
Kenneth G. Caulton is an inorganic chemist who works on, and has made significant contributions to, projects dealing with transition metal hydrides. He is currently Distinguished Professor at Indiana University. Specifically, Caulton has worked on the chemistry of paramagnetic organometallic complexes, metal polyhydride complexes and the dihydrogen ligand, catalytic activation of carbon monoxide and carbon dioxide, and alkoxide chemistry. Caulton's work with transition metal complexes is ultimately aimed to create complexes that exhibit unexpected and novel reactivities.
T. Don Tilley is a Professor of Chemistry at the University of California, Berkeley.
In homogeneous catalysis, C2-symmetric ligands refer to ligands that lack mirror symmetry but have C2 symmetry. Such ligands are usually bidentate and are valuable in catalysis. The C2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. C2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including C2-symmetric ligands, combine with metals or other groups to form chiral catalysts. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product.










