Related Research Articles
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of a molecule. Chemical shifts are also used to describe signals in other forms of spectroscopy such as photoemission spectroscopy.
CIDNP, often pronounced like "kidnip", is a nuclear magnetic resonance (NMR) technique that is used to study chemical reactions that involve radicals. It detects the non-Boltzmann (non-thermal) nuclear spin state distribution produced in these reactions as enhanced absorption or emission signals.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. This spectroscopy is based on the measurement of absorption of electromagnetic radiations in the radio frequency region from roughly 4 to 900 MHz. Absorption of radio waves in the presence of magnetic field is accompanied by a special type of nuclear transition, and for this reason, such type of spectroscopy is known as Nuclear Magnetic Resonance Spectroscopy. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds.

Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford.

Solid-state NMR (ssNMR) spectroscopy is a technique for characterizing atomic level structure in solid materials e.g. powders, single crystals and amorphous samples and tissues using nuclear magnetic resonance (NMR) spectroscopy. The anisotropic part of many spin interactions are present in solid-state NMR, unlike in solution-state NMR where rapid tumbling motion averages out many of the spin interactions. As a result, solid-state NMR spectra are characterised by larger linewidths than in solution state NMR, which can be utilized to give quantitative information on the molecular structure, conformation and dynamics of the material. Solid-state NMR is often combined with magic angle spinning to remove anisotropic interactions and improve the resolution as well as the sensitivity of the technique.
In nuclear chemistry and nuclear physics, J-couplings are mediated through chemical bonds connecting two spins. It is an indirect interaction between two nuclear spins that arises from hyperfine interactions between the nuclei and local electrons. In NMR spectroscopy, J-coupling contains information about relative bond distances and angles. Most importantly, J-coupling provides information on the connectivity of chemical bonds. It is responsible for the often complex splitting of resonance lines in the NMR spectra of fairly simple molecules.
In 1957, the research organization of the Chemicals Department of E. I. du Pont de Nemours and Company was renamed Central Research Department, beginning the history of the premier scientific organization within DuPont and one of the foremost industrial laboratories devoted to basic science. Located primarily at the DuPont Experimental Station and Chestnut Run, in Wilmington, Delaware, it has expanded to include laboratories in Geneva, Switzerland, Seoul, South Korea, Shanghai, China, and India(Hyderabad). In January, 2016 a major layoff marked the end of the organization.
During nuclear magnetic resonance observations, spin–lattice relaxation is the mechanism by which the longitudinal component of the total nuclear magnetic moment vector (parallel to the constant magnetic field) exponentially relaxes from a higher energy, non-equilibrium state to thermodynamic equilibrium with its surroundings (the "lattice"). It is characterized by the spin–lattice relaxation time, a time constant known as T1.
Herbert Sander Gutowsky was an American chemist who was a professor of chemistry at the University of Illinois Urbana-Champaign. Gutowsky was the first to apply nuclear magnetic resonance (NMR) methods to the field of chemistry. He used nuclear magnetic resonance spectroscopy to determine the structure of molecules. His pioneering work developed experimental control of NMR as a scientific instrument, connected experimental observations with theoretical models, and made NMR one of the most effective analytical tools for analysis of molecular structure and dynamics in liquids, solids, and gases, used in chemical and medical research, His work was relevant to the solving of problems in chemistry, biochemistry, and materials science, and has influenced many of the subfields of more recent NMR spectroscopy.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI).
Electron nuclear double resonance (ENDOR) is a magnetic resonance technique for elucidating the molecular and electronic structure of paramagnetic species. The technique was first introduced to resolve interactions in electron paramagnetic resonance (EPR) spectra. It is currently practiced in a variety of modalities, mainly in the areas of biophysics and heterogeneous catalysis.
Dennis Frederick Evans was an English chemist who made important contributions to nuclear magnetic resonance, magnetochemistry and other aspects of chemistry.

David Lyndon Emsley FRSC is a British chemist specialising in solid-state nuclear magnetic resonance and a professor at EPFL. He was awarded the 2012 Grand Prix Charles-Leopold Mayer of the French Académie des Sciences and the 2015 Bourke Award of the Royal Society of Chemistry.

The Journal of Magnetic Resonance (JMR) is a monthly peer-reviewed scientific journal that publishes original research in the field of magnetic resonance, including nuclear magnetic resonance, electron paramagnetic resonance, magnetic resonance imaging, magnetic resonance spectroscopy and nuclear quadrupole resonance. Since 2021, its editor-in-chief has been Tatyana Polenova of the University of Delaware. According to the Journal Citation Reports, it has an impact factor of 2.624. Authors can pay a fee to have their articles published as open access.
Sandra Eaton is an American chemist and Professor at the University of Denver, known for her work on electron paramagnetic resonance.
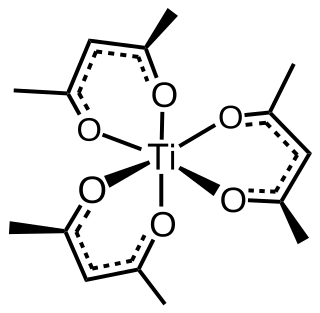
Tris(acetylacetonato)titanium(III), often abbreviated Ti(acac)3, is a coordination complex of titanium(III) featuring acetylacetonate (acac) ligands, making it one of a family of metal acetylacetonates. It is a blue air-sensitive solid that dissolves in nonpolar organic solvents. The compound is prepared by treating titanium trichloride with acetylacetone in the presence of base. Being paramagnetic, it gives a contact-shifted proton NMR signal at 60 ppm upfield of TMS assigned to the methyl group.
R. David Britt is the Winston Ko Chair and Distinguished Professor of Chemistry at the University of California, Davis. Britt uses electron paramagnetic resonance (EPR) spectroscopy to study metalloenzymes and enzymes containing organic radicals in their active sites. Britt is the recipient of multiple awards for his research, including the Bioinorganic Chemistry Award in 2019 and the Bruker Prize in 2015 from the Royal Society of Chemistry. He has received a Gold Medal from the International EPR Society (2014), and the Zavoisky Award from the Kazan Scientific Center of the Russian Academy of Sciences (2018). He is a Fellow of the American Association for the Advancement of Science and of the Royal Society of Chemistry.
Jeffrey Allen Reimer is an American chemist, academic, author and researcher. He is the C. Judson King Endowed Professor, a Warren and Katharine Schlinger Distinguished Professor and the Chair of the Chemical and Biomolecular Engineering Department at University of California, Berkeley.
Frances Ann Walker was an American chemist known for her work on heme protein chemistry. She was an elected fellow of the American Association for the Advancement of Science and the American Chemical Society.

Eric Oldfield is a British chemist, the Harriet A. Harlin Professor of Chemistry and a Professor of Biophysics at the University of Illinois at Urbana-Champaign. He is known for his work in nuclear magnetic resonance spectroscopy of lipids, proteins, and membranes; of inorganic solids; in computational chemistry, and in microbiology and parasitology. He is a member of the American Association for the Advancement of Science, a Fellow of the Royal Society of Chemistry, and a Fellow of the American Physical Society.
References
- ↑ Phillips, W. D.; Looney, C. E. A nuclear magnetic resonance study of rotational isomerism in alkyl nitrites. Mol. Spectroscopy (1957), 1 35-42.
- ↑ Phillips, W. D.. Restricted rotation in amides as evidenced by nuclear magnetic resonance. Journal of Chemical Physics (1955), 23 1363-4.
- ↑ Phillips, W. D.. Studies of hindered internal rotation in organic molecules by nuclear magnetic resonance. Annals of the New York Academy of Sciences (1958), 70 817-32.
- ↑ Looney, Catharine E.; Phillips, W. D.; Reilly, E. L. Nuclear magnetic resonance and infrared study of hindered rotation in nitrosamines. Journal of the American Chemical Society (1957), 79 6136-42.
- ↑ Drysdale, J. J.; Philips, W. D.. Restricted rotation in substituted ethanes as evidenced by nuclear magnetic resonance. Journal of the American Chemical Society (1957), 79 319-22.
- ↑ Muetterties, E. L.; Phillips, W. D.. Structure of ClF3 and exchange studies on some halogen fluorides by nuclear magnetic resonance (n.m.r.). Journal of the American Chemical Society (1957), 79 322-6.
- ↑ Muetterties, E. L.; Phillips, W. D.. Fluoroarsenites. Journal of the American Chemical Society (1957), 79 3686-7.
- ↑ Muetterties, E. L.; Phillips, W. D.. Lewis character of tellurium hexafluoride. Journal of the American Chemical Society (1957), 79 2975.
- ↑ Phillips, W. D.; Miller, H. C.; Muetterties, E. L. B11 magnetic resonance study of boron compounds. Journal of the American Chemical Society (1959), 81 4496-500.
- ↑ Muetterties, E. L.; Phillips, W. D.. Structure of exchange processes in some inorganic fluorides by nuclear magnetic resonance. Journal of the American Chemical Society (1959), 81 1084-8.
- ↑ Muetterties, Earl L.; Phillips, William Dale. Fluorine exchange in sulfur tetrafluoride. Journal of Chemical Physics (1967), 46(7), 2861-2.
- ↑ Phillips, W. D.. Fluorine magnetic resonance spectra of some mono- and disubstituted tetrafluorocyclobutanes. J. Chem. Phys. (1956), 25 949-55.
- ↑ Eaton, D. R.; Josey, A. D.; Phillips, W. D.; Benson, R. E. F19 contact interaction shifts: effects on fluorine conjugation. Molecular Physics (1962), 5 407-16.
- ↑ Phillips, W. D.. High-resolution H1 and F19 magnetic resonance spectra of organic molecules. Detn. Org. Struct. Phys. Methods (1961), 2 401-63.
- ↑ Merriefield, R. E.; Phillips, W. D.. Cyanocarbon chemistry. II. Spectroscopic studies of the molecular complexes of tetracyanoethylene. Journal of the American Chemical Society (1958), 80 2778-82.
- ↑ Chesnut, D. B.; Foster, H.; Phillips, W. D.. EPR (electron paramagnetic resonance) studies of spin correlation in some ion radical salts. Journal of Chemical Physics (1961), 34 684-5.
- ↑ Phillips, W. D.; Rowell, J. C. Electron spin paramagnetic resonance (EPR) studies of the tetracyanoethylene anion radical. Journal of Chemical Physics (1960), 33 626-7.
- ↑ Goll, R. J.; Phillips, W. D.. Pressure dependence of the methyltriphenylphosphonium (TCNQ)2 phase change. Journal of Chemical Physics (1965), 43(3), 1076.
- ↑ Boyd, Richard H.; Phillips, William D.. Solution dimerization of the tetracyanoquinodimethan ion radical. Journal of Chemical Physics (1965), 43(9), 2927-9.
- ↑ LaLancette, E. A.; Eaton, D. R.; Benson, R. E.; Phillips, W. D.. N.M.R. (nuclear magnetic resonance) contact shifts in paramagnetic Ni(II) salicylaldimines. Journal of the American Chemical Society (1962), 84 3968-70.
- ↑ Eaton, D. R.; Josey, A. D.; Benson, R. E.; Phillips, W. D.; Cairns, T. L. Unpaired electron distribution in π-systems. Journal of the American Chemical Society (1962), 84 4100-6.
- ↑ Eaton, D. R.; Phillips, W. D.; Caldwell, D. J. Configurations and magnetic properties of the nickel(II) aminotroponeimineates. Journal of the American Chemical Society (1963), 85 397-406.
- ↑ Eaton, D. R.; Josey, A. D.; Phillips, W. D.; Benson, R. E. Hyperfine contact shifts and spin density distributions in some Ni(II) chelates. Discussions of the Faraday Society (1962), No. 34 77-87.
- ↑ Eaton, D. R.; Josey, A. D.; Phillips, W. D.; Benson, R. E. Spin-density distributions in conjugated ligands of paramagnetic chelates from nuclear magnetic resonance contact interaction shifts. Journal of Chemical Physics (1962), 37 347-60.
- ↑ McDonald, C. C.; Phillips, W. D.; Mower, H. F. An electron spin resonance study of some complexes of iron, nitric oxide, and anionic ligands. Journal of the American Chemical Society (1965), 87(15), 3319-26.
- ↑ Eaton, D. R.; Phillips, W. D.. Spin delocalization in mixed tetrahedral Ni (II) complexes. Journal of Chemical Physics (1965), 43(2), 392-8.
- ↑ McDonald, C. C.; Phillips, W. D.. A nuclear magnetic resonance (N.M.R.) study of structures of cobalt(II)-histidine complexes. Journal of the American Chemical Society (1963), 85(23), 3736-42.
- ↑ Eaton, D. R.; Phillips, W. D.. Nuclear magnetic resonance of paramagnetic molecules. Advan. Magn. Resonance (1966), 1 103-48.
- ↑ McDonald, Charles C.; Phillips, William Dale; Vinogradov, Serge N. Proton magnetic resonance evidence for methionine-iron coordination in mammalian-type ferrocytochrome c. Biochemical and Biophysical Research Communications (1969), 36(3), 442-9.
- ↑ Blomstrom, D. C.; Knight, E. Jr.; Phillips, W. D.; Weiher, J. F. The nature of Fe in ferredoxin. Proceedings of the National Academy of Sciences of the United States of America (1964), 51(6), 1085-92.
- ↑ Poe, Martin; Phillips, William Dale; McDonald, Charles C.; Lovenberg, Walter. Proton magnetic resonance study of ferredoxin from Clostridium pasteurianum. Proceedings of the National Academy of Sciences of the United States of America (1970), 65(4), 797-804.
- ↑ Poe, Martin; Phillips, William Dale; McDonald, Charles C.; Orme-Johnson, William H. PMR and magnetic susceptibility studies on Clostridium acidi-urici ferredoxin. Biochemical and Biophysical Research Communications (1971), 42(4), 705-13.
- ↑ Glickson, J. D.; Phillips, William Dale; McDonald, Charles C.; Poe, M. PMR characterization of alfalfa and soybean ferredoxins: the existence of two ferredoxins in soybean. Biochemical and Biophysical Research Communication (1971), 42(2), 271-9.
- ↑ Poe, Martin; Phillips, William Dale; Glickson, J. D.; McDonald, Charles C.; San Pietro, Anthony. Proton magnetic resonance studies of the ferredoxins from spinach and parsley. Proceedings of the National Academy of Sciences of the United States of America (1971), 68(1), 68-71.
- ↑ Phillips, W. D.; Poe, Martin. NMR spectroscopy of the iron-sulfur proteins. Iron-Sulfur Proteins (1973), 2 255-84.
- ↑ Phillips, William Dale; Poe, M.; Weiher, J. F.; McDonald, Charles C.; Lovenberg, W. Proton magnetic resonance, magnetic susceptibility, and Moessbauer studies of Clostridium pasteurianum rubredoxin. Nature (London, United Kingdom) (1970), 227(5258), 574-7.
- ↑ Phillips, W. D.; Poe, Martin. Contact shifts and magnetic susceptibilities in iron-sulfur proteins as determined from nuclear magnetic resonance spectra. Methods Enzymol. (1972), 24(Pt. B), 304-17.
- ↑ McDonald, C. C.; Phillips, W. D.; Lovenberg, W.; Holm, R. H. PMR studies on Clostridium pasteurianum ferredoxin. Origins of contact-shifted resonances and denaturation by dimethyl sulfoxide. Annals of the New York Academy of Sciences (1973), 222 789-99.
- ↑ Phillips, William D.; McDonald, C. C.; Stombaugh, N. A.; Orme-Johnson, W. H. Proton magnetic resonance and magnetic susceptibility characterization of ferredoxin I from Bacillus polymyxa. Proceedings of the National Academy of Sciences of the United States of America (1974), 71(1), 140-3.
- ↑ Glickson, J. D.; McDonald, Charles C.; Phillips, William Dale. Assignment of tryptophan indole NH Proton resonances of lysozyme. Biochemical and Biophysical Research Communications (1969), 35(4), 492-8.
- ↑ McDonald, Charles C.; Phillips, William Dale. Perturbation of the PMR [proton magnetic resonance] spectrum of lysozyme by cobaltous ions. Biochemical and Biophysical Research Communications (1969), 35(1), 43-51.
- ↑ Phillips, W. D.; Glickson, J. D.; Rupley, J. A. Proton magnetic resonance study of the indole NH resonances of lysozyme. Assignment, deuterium exchange kinetics, and inhibitor binding. Journal of the American Chemical Society (1971), 93(16), 4031-8.
- ↑ McDonald, Charles C.; Phillips, William Dale; Glickson, J. D. Nuclear magnetic resonance study of the mechanism of reversible denaturation of lysozyme. Journal of the American Chemical Society (1971), 93(1), 235-46.
- ↑ McDonald, C. C.; Phillips, W. D.; Penman, Sheldon. Nucleic acids; a nuclear magnetic resonance (NMR) study. Science (Washington, DC, United States) (1964), 144(3623), 1234-7.
- ↑ McDonald, Charles C.; Phillips, William Dale; Lazar, Joseph. Nuclear magnetic resonance determination of thymine nearest neighbor base frequency ratios in deoxyribonucleic acid. Journal of the American Chemical Society (1967), 89(16), 4166-70.
- ↑ McDonald, C. C.; Phillips, W. D.; Penswick, John. N.M.R study of the secondary structure of sRNA. Biopolymers (1965), 3(6), 609-16.
- ↑ McDonald, Charles C.; Phillips, William D.. Proton magnetic resonance spectra of proteins in random-coil configurations. Journal of the American Chemical Society (1969), 91(6), 1513-21.
- ↑ McDonald, Charles C.; Phillips, William Dale. Nuclear magnetic resonance studies of biological macromolecules. Magn. Resonance Biol. Syst., Proc. Int. Conf., 2nd, Stockholm (1967), Volume Date 1966, 3-23.
- ↑ McDonald, Charles C.; Phillips, William Dale. Manifestations of the tertiary structures of proteins in high-frequency nuclear magnetic resonance. Journal of the American Chemical Society (1967), 89(24), 6332-41.
- ↑ Panar, M.; Phillips, W. D.. Magnetic ordering of poly(γ-benzyl L-glutamate) solutions. Journal of the American Chemical Society (1968), 90(14), 3880-2.
- ↑ Phillips, W. D.. Biological applications of NMR. NMR Paramagn. Mol. (1973), 421-78.