
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

Boron hydride clusters are compounds with the formula BxHy or related anions, where x ≥ 3. Many such cluster compounds are known. Common examples are those with 5, 10, and 12 boron atoms. Although they have few practical applications, the borane hydride clusters exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes are also well developed.

Decaborane, also called decaborane(14), is the inorganic compound with the chemical formula B10H14. It is classified as a borane and more specifically a boron hydride cluster. This white crystalline compound is one of the principal boron hydride clusters, both as a reference structure and as a precursor to other boron hydrides. It is toxic and volatile, giving off a foul odor, like that of burnt rubber or chocolate.
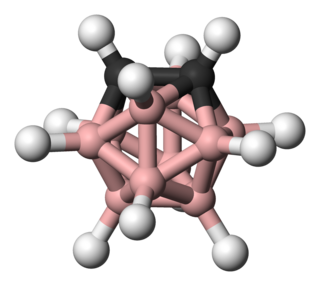
Carboranes are electron-delocalized clusters composed of boron, carbon and hydrogen atoms. Like many of the related boron hydrides, these clusters are polyhedra or fragments of polyhedra. Carboranes are one class of heteroboranes.
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found as counterions for cationic metal complexes with an unsaturated coordination sphere. These special anions are essential components of homogeneous alkene polymerisation catalysts, where the active catalyst is a coordinatively unsaturated, cationic transition metal complex. For example, they are employed as counterions for the 14 valence electron cations [(C5H5)2ZrR]+ (R = methyl or a growing polyethylene chain). Complexes derived from non-coordinating anions have been used to catalyze hydrogenation, hydrosilylation, oligomerization, and the living polymerization of alkenes. The popularization of non-coordinating anions has contributed to increased understanding of agostic complexes wherein hydrocarbons and hydrogen serve as ligands. Non-coordinating anions are important components of many superacids, which result from the combination of Brønsted acids and Lewis acids.

Organoboron chemistry or organoborane chemistry studies organoboron compounds, also called organoboranes. These chemical compounds combine boron and carbon; typically, they are organic derivatives of borane (BH3), as in the trialkyl boranes.
Marion Frederick Hawthorne was an inorganic chemist who made contributions to the chemistry of boron hydrides, especially their clusters.

Tris(pentafluorophenyl)borane, sometimes referred to as "BCF", is the chemical compound (C6F5)3B. It is a white, volatile solid. The molecule consists of three pentafluorophenyl groups attached in a "paddle-wheel" manner to a central boron atom; the BC3 core is planar. It has been described as the “ideal Lewis acid” because of its high thermal stability and the relative inertness of the B-C bonds. Related fluoro-substituted boron compounds, such as those containing B−CF3 groups, decompose with formation of B-F bonds. Tris(pentafluorophenyl)borane is thermally stable at temperatures well over 200 °C, resistant to oxygen, and water-tolerant.
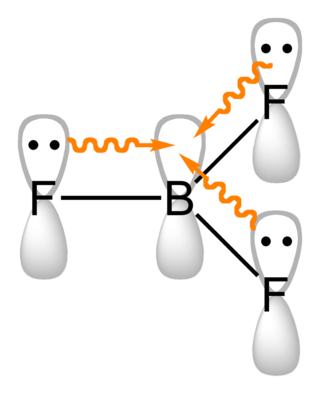
Boron compounds are compounds containing the element boron. In the most familiar compounds, boron has the formal oxidation state +3. These include oxides, sulfides, nitrides, and halides.
In 1957, the research organization of the Chemicals Department of E. I. du Pont de Nemours and Company was renamed Central Research Department, beginning the history of the premier scientific organization within DuPont and one of the foremost industrial laboratories devoted to basic science. Located primarily at the DuPont Experimental Station and Chestnut Run, in Wilmington, Delaware, it expanded to include laboratories in Geneva, Switzerland, Seoul, South Korea, Shanghai, China, and India (Hyderabad). In January, 2016 a major layoff marked the end of the organization.
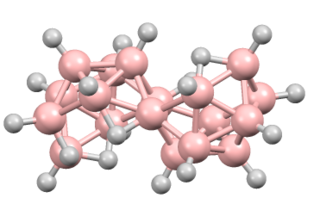
Octadecaborane is an inorganic compound, a boron hydride cluster with chemical formula B18H22. It is a colorless flammable solid, like many higher boron hydrides. Although the compound has no practical applications, its structure is of theoretical and pedagogical interest.

Caesium dodecaborate is an inorganic compound with the formula Cs2B12H12. It is a salt composed of caesium and dodecaborate(12) ions. The [B12H12]2− anion has been of great theoretical interest to the chemistry community.
John David Kennedy is a chemist and emeritus professor of inorganic chemistry at the University of Leeds. He works in the area of polyhedral borane chemistry.

Eluvathingal Devassy Jemmis is a professor of theoretical chemistry at the Indian Institute of Science, Bangalore, India. He was the founding director of Indian Institute of Science Education and Research, Thiruvananthapuram (IISER-TVM). His primary area of research is applied theoretical chemistry with emphasis on structure, bonding and reactivity, across the periodic table of the elements. Apart from many of his contributions to applied theoretical chemistry, an equivalent of the structural chemistry of carbon, as exemplified by the Huckel 4n+2 Rule, benzenoid aromatics and graphite, and tetrahedral carbon and diamond, is brought in the structural chemistry of boron by the Jemmis mno rules which relates polyhedral and macropolyhedral boranes to allotropes of boron and boron-rich solids. He has been awarded Padma Shri in Science and Engineering category by the Government of India.
Frederick Nye Tebbe was a chemist known for his work on organometallic chemistry. Tebbe was born in Oakland, California on March 20, 1935. His father, Charles L. Tebbe, worked for the United States Forest Service so Fred’s early education took place in Montana, Oregon, Maryland and Pennsylvania. He married Margaret Manzer in 1960, and they had a son and a daughter. He died of pancreatic cancer at his home in Delaware on September 28, 1995.
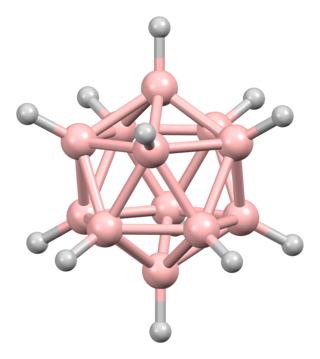
The dodecaborate(12) anion, [B12H12]2−, is a borane with an icosahedral arrangement of 12 boron atoms, with each boron atom being attached to a hydrogen atom. Its symmetry is classified by the molecular point group Ih.

1,2-Dimethyldiborane is an organoboron compound with the formula [(CH3)BH2]2. Structurally, it is related to diborane, but with methyl groups replacing terminal hydrides on each boron. It is the dimer of methylborane, CH3BH2, the simplest alkylborane. 1,2-Dimethyldiborane can exist in a cis- and a trans arrangement. 1,2-Dimethyldiborane is an easily condensed, colorless gas that ignites spontaneously in air.

Trimethyldiborane, (CH3)3B2H3 is a molecule containing boron carbon and hydrogen. It is an alkylborane, consisting of three methyl group substituted for a hydrogen in diborane. It can be considered a mixed dimer: (CH3)2BH2BH(CH3) or dimethylborane and methylborane. called 1,2-dimethyldiborane. Other combinations of methylation occur on diborane, including monomethyldiborane, 1,2-dimethyldiborane, tetramethyldiborane, 1,1-dimethylborane and trimethylborane. At room temperature the substance is at equilibrium between these forms, so it is difficult to keep it pure. The methylboranes were first prepared by H. I. Schlesinger and A. O. Walker in the 1930s.

Borane carbonyl is the inorganic compound with the formula H3BCO. This colorless gas is the adduct of borane and carbon monoxide. It is usually prepared by combining borane-ether complexes and CO. The compound is mainly of theoretical and pedagogical interest.














