Nitrate is a polyatomic ion with the chemical formula NO−
3. Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble nitrate is bismuth oxynitrate.

Potassium ferrocyanide is the inorganic compound with formula K4[Fe(CN)6]·3H2O. It is the potassium salt of the coordination complex [Fe(CN)6]4−. This salt forms lemon-yellow monoclinic crystals.

Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula KNO
3. It is an ionic salt of potassium ions K+ and nitrate ions NO3−, and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or nitre in the UK). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpeter (or saltpetre in the UK).

Potassium chloride is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt-like taste. Potassium chloride can be obtained from ancient dried lake deposits. KCl is used as a fertilizer, in medicine, in scientific applications, domestic water softeners, and in food processing, where it may be known as E number additive E508.

Sodium nitrate is the chemical compound with the formula NaNO
3. This alkali metal nitrate salt is also known as Chile saltpeter to distinguish it from ordinary saltpeter, potassium nitrate. The mineral form is also known as nitratine, nitratite or soda niter.
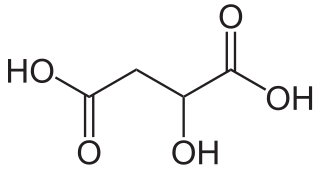
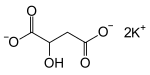
Malic acid is an organic compound with the molecular formula C4H6O5. It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms, though only the L-isomer exists naturally. The salts and esters of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle.

Sodium nitrite is an inorganic compound with the chemical formula NaNO2. It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. From an industrial perspective, it is the most important nitrite salt. It is a precursor to a variety of organic compounds, such as pharmaceuticals, dyes, and pesticides, but it is probably best known as a food additive used in processed meats and (in some countries) in fish products.

Plant nutrition is the study of the chemical elements and compounds necessary for plant growth and reproduction, plant metabolism and their external supply. In its absence the plant is unable to complete a normal life cycle, or that the element is part of some essential plant constituent or metabolite. This is in accordance with Justus von Liebig’s law of the minimum. The total essential plant nutrients include seventeen different elements: carbon, oxygen and hydrogen which are absorbed from the air, whereas other nutrients including nitrogen are typically obtained from the soil.
Nitrogen assimilation is the formation of organic nitrogen compounds like amino acids from inorganic nitrogen compounds present in the environment. Organisms like plants, fungi and certain bacteria that can fix nitrogen gas (N2) depend on the ability to assimilate nitrate or ammonia for their needs. Other organisms, like animals, depend entirely on organic nitrogen from their food.

Processed meat is considered to be any meat which has been modified in order to either improve its taste or to extend its shelf life. Methods of meat processing include salting, curing, fermentation, smoking, boiling, frying, and/or the addition of chemical preservatives. Processed meat is usually composed of pork or beef, but also poultry, while it can also contain offal or meat by-products such as blood. Processed meat products include bacon, ham, sausages, salami, corned beef, jerky, hot dogs, lunch meat, canned meat, chicken nuggets, and meat-based sauces. Meat processing includes all the processes that change fresh meat with the exception of simple mechanical processes such as cutting, grinding or mixing.
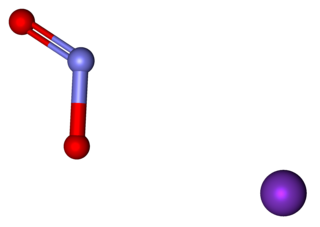
Potassium nitrite (distinct from potassium nitrate) is the inorganic compound with the chemical formula KNO2. It is an ionic salt of potassium ions K+ and nitrite ions NO2−, which forms a white or slightly yellow, hygroscopic crystalline powder that is soluble in water.
E350 is an EU recognised food additive. It comes in two forms,

Potassium acetate (also called potassium ethanoate), (CH3COOK) is the potassium salt of acetic acid. It is a hygroscopic solid at room temperature.

Curing salt is used in meat processing to generate a pinkish shade and to extend shelf life. It is both a color agent and a means to facilitate food preservation as it prevents or slows spoilage by bacteria or fungus. Curing salts are generally a mixture of sodium chloride and sodium nitrite, and are used for pickling meats as part of the process to make sausage or cured meat such as ham, bacon, pastrami, corned beef, etc. Though it has been suggested that the reason for using nitrite-containing curing salt is to prevent botulism, a 2018 study by the British Meat Producers Association determined that legally permitted levels of nitrite have no effect on the growth of the Clostridium botulinum bacteria that causes botulism, in line with the UK's Advisory Committee on the Microbiological Safety of Food opinion that nitrites are not required to prevent C. botulinum growth and extend shelf life..

Sociedad Química y Minera de Chile (SQM) is a Chilean chemical company and a supplier of plant nutrients, iodine, lithium and industrial chemicals. It is the world’s biggest lithium producer.

Cured fish is fish which has been cured by subjecting it to fermentation, pickling, smoking, or some combination of these before it is eaten. These food preservation processes can include adding salt, nitrates, nitrite or sugar, can involve smoking and flavoring the fish, and may include cooking it. The earliest form of curing fish was dehydration. Other methods, such as smoking fish or salt-curing also go back for thousands of years. The term "cure" is derived from the Latin curare, meaning to take care of. It was first recorded in reference to fish in 1743.
Ammonium malate refers to organic compounds containing malate and ammonium. Two stoichiometries are discussed: NH4H(C2H3OH(CO2)2) with one ammonium ion per formula unit, and (NH4)2(C2H3OH(CO2)2). Malate, the conjugate base of malic acid, is chiral. Consequently a variety of salts are possible, R vs S vs racemic. The monoammonium salt has been crystallized as the monohydrate.

Sodium malate is a compound with formula Na2(C2H4O(COO)2). It is the sodium salt of malic acid. As a food additive, it has the E number E350.

Calcium malate is a compound with formula Ca(C2H4O(COO)2). It is the calcium salt of malic acid. As a food additive, it has the E number E352.