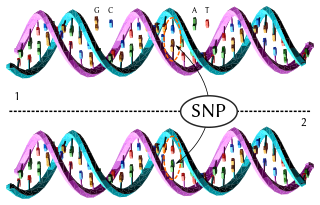
In genetics, a single-nucleotide polymorphism is a germline substitution of a single nucleotide at a specific position in the genome and is present in a sufficiently large fraction of the population. Single nucleotide substitutions with an allele frequency of less than 1% are called "single-nucleotide variants", not SNPs.
In molecular biology, SNP array is a type of DNA microarray which is used to detect polymorphisms within a population. A single nucleotide polymorphism (SNP), a variation at a single site in DNA, is the most frequent type of variation in the genome. Around 335 million SNPs have been identified in the human genome, 15 million of which are present at frequencies of 1% or higher across different populations worldwide.
A tag SNP is a representative single nucleotide polymorphism (SNP) in a region of the genome with high linkage disequilibrium that represents a group of SNPs called a haplotype. It is possible to identify genetic variation and association to phenotypes without genotyping every SNP in a chromosomal region. This reduces the expense and time of mapping genome areas associated with disease, since it eliminates the need to study every individual SNP. Tag SNPs are useful in whole-genome SNP association studies in which hundreds of thousands of SNPs across the entire genome are genotyped.
The common disease-common variant hypothesis predicts that common disease-causing alleles, or variants, will be found in all human populations which manifest a given disease. Common variants are known to exist in coding and regulatory sequences of genes. According to the CD-CV hypothesis, some of those variants lead to susceptibility to complex polygenic diseases. Each variant at each gene influencing a complex disease will have a small additive or multiplicative effect on the disease phenotype. These diseases, or traits, are evolutionarily neutral in part because so many genes influence the traits. The hypothesis has held in the case of putative causal variants in apolipoprotein E, including APOE ε4, associated with Alzheimer's disease. IL23R has been found to be associated with Crohn's disease; the at-risk allele has a frequency 93% in the general population.

In genomics, a genome-wide association study, is an observational study of a genome-wide set of genetic variants in different individuals to see if any variant is associated with a trait. GWA studies typically focus on associations between single-nucleotide polymorphisms (SNPs) and traits like major human diseases, but can equally be applied to any other genetic variants and any other organisms.
In multivariate quantitative genetics, a genetic correlation is the proportion of variance that two traits share due to genetic causes, the correlation between the genetic influences on a trait and the genetic influences on a different trait estimating the degree of pleiotropy or causal overlap. A genetic correlation of 0 implies that the genetic effects on one trait are independent of the other, while a correlation of 1 implies that all of the genetic influences on the two traits are identical. The bivariate genetic correlation can be generalized to inferring genetic latent variable factors across > 2 traits using factor analysis. Genetic correlation models were introduced into behavioral genetics in the 1970s–1980s.
Expression quantitative trait loci (eQTLs) are genomic loci that explain variation in expression levels of mRNAs.
In genetics, association mapping, also known as "linkage disequilibrium mapping", is a method of mapping quantitative trait loci (QTLs) that takes advantage of historic linkage disequilibrium to link phenotypes to genotypes, uncovering genetic associations.

Exome sequencing, also known as whole exome sequencing (WES), is a genomic technique for sequencing all of the protein-coding regions of genes in a genome. It consists of two steps: the first step is to select only the subset of DNA that encodes proteins. These regions are known as exons—humans have about 180,000 exons, constituting about 1% of the human genome, or approximately 30 million base pairs. The second step is to sequence the exonic DNA using any high-throughput DNA sequencing technology.
The missing heritability problem is the fact that single genetic variations cannot account for much of the heritability of diseases, behaviors, and other phenotypes. This is a problem that has significant implications for medicine, since a person's susceptibility to disease may depend more on the combined effect of all the genes in the background than on the disease genes in the foreground, or the role of genes may have been severely overestimated.
Single nucleotide polymorphism annotation is the process of predicting the effect or function of an individual SNP using SNP annotation tools. In SNP annotation the biological information is extracted, collected and displayed in a clear form amenable to query. SNP functional annotation is typically performed based on the available information on nucleic acid and protein sequences.

Michael Edward "Mike" Goddard is a professorial fellow in animal genetics at the University of Melbourne, Australia.

In genetics, a polygenic score (PGS), also called a polygenic index (PGI), polygenic risk score (PRS), genetic risk score, or genome-wide score, is a number that summarizes the estimated effect of many genetic variants on an individual's phenotype, typically calculated as a weighted sum of trait-associated alleles. It reflects an individual's estimated genetic predisposition for a given trait and can be used as a predictor for that trait. In other words, it gives an estimate of how likely an individual is to have a given trait only based on genetics, without taking environmental factors into account. Polygenic scores are widely used in animal breeding and plant breeding due to their efficacy in improving livestock breeding and crops. In humans, polygenic scores are typically generated from genome-wide association study (GWAS) data.
Gene Relationships Across Implicated Loci(GRAIL) is a free web application developed by Soumya Raychaudhuri at the Broad Institute with the goal of determining the relationships among genes in different disease associated loci through statistical analysis.
Naomi Ruth Wray is an Australian statistical geneticist at the University of Queensland, where she is a Professorial Research Fellow at the Institute for Molecular Bioscience and an Affiliate Professor in the Queensland Brain Institute. She is also a National Health and Medical Research Council (NHMRC) Principal Research Fellow and, along with Peter Visscher and Jian Yang, is one of the three executive team members of the NHMRC-funded Program in Complex Trait Genomics.

ANNOVAR is a bioinformatics software tool for the interpretation and prioritization of single nucleotide variants (SNVs), insertions, deletions, and copy number variants (CNVs) of a given genome. It has the ability to annotate human genomes hg18, hg19, hg38, and model organisms genomes such as: mouse, zebrafish, fruit fly, roundworm, yeast and many others. The annotations could be used to determine the functional consequences of the mutations on the genes and organisms, infer cytogenetic bands, report functional importance scores, and/or find variants in conserved regions. ANNOVAR along with SNP effect (SnpEFF) and Variant Effect Predictor (VEP) are three of the most commonly used variant annotation tools.
The GWAS catalog is a free online database that compiles data of genome-wide association studies (GWAS), summarizing unstructured data from different literature sources into accessible high quality data. It was created by the National Human Genome Research Institute (NHGRI) in 2008 and have become a collaborative project between the NHGRI and the European Bioinformatics Institute (EBI) since 2010. As of September 2018, it has included 71,673 SNP–trait associations in 3,567 publications.
Impute.me. was an open-source non-profit web application that allowed members of the public to use their data from direct-to-consumer (DTC) genetic tests to calculate polygenic risk scores (PRS) for complex diseases and cognitive and personality traits. In July 2022, Lasse Folkerson, initiator and operator of impute.me, took the website offline.

N-Acetylated Alpha-Linked Acidic Dipeptidase Like 2 (NAALADL2) is a protein, encoded by the gene NAALADL2 in humans. NAALADL2 shares 25%–26% sequence identity and 45% sequence similarity with the glutamate carboxypeptidase II family which includes prostate cancer marker PSMA (FOLH1/NAALAD1). The NAALADL2 gene is a giant gene spanning 1.37 Mb which is approximately 49 times larger than the average gene size of 28 kb. Gene length is correlated with the number of transcript variants of a gene, as such, NAALADL2 undergoes extensive alternative splicing and has 12 splice variants as defined by Ensembl.

Andre Franke, born on 16 October 1978, is a geneticist, academic, and university professor. He is a Full W3 Professor of Molecular Medicine at the Christian-Albrechts-University of Kiel, and a managing director at the Institute of Clinical Molecular Biology.











