
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of over 100 μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm.

Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton. Microtubules function in many essential cellular processes, including mitosis. Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division.
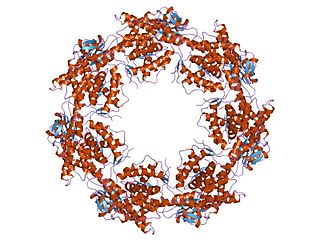
HSP60, also known as chaperonins (Cpn), is a family of heat shock proteins originally sorted by their 60kDa molecular mass. They prevent misfolding of proteins during stressful situations such as high heat, by assisting protein folding. HSP60 belong to a large class of molecules that assist protein folding, called molecular chaperones.

A DNA clamp, also known as a sliding clamp, is a protein complex that serves as a processivity-promoting factor in DNA replication. As a critical component of the DNA polymerase III holoenzyme, the clamp protein binds DNA polymerase and prevents this enzyme from dissociating from the template DNA strand. The clamp-polymerase protein–protein interactions are stronger and more specific than the direct interactions between the polymerase and the template DNA strand; because one of the rate-limiting steps in the DNA synthesis reaction is the association of the polymerase with the DNA template, the presence of the sliding clamp dramatically increases the number of nucleotides that the polymerase can add to the growing strand per association event. The presence of the DNA clamp can increase the rate of DNA synthesis up to 1,000-fold compared with a nonprocessive polymerase.

In humans, the gene T-complex 1, a.k.a. TCP1, encodes the protein TCP-1, a.k.a. T-complex protein 1 subunit alpha.
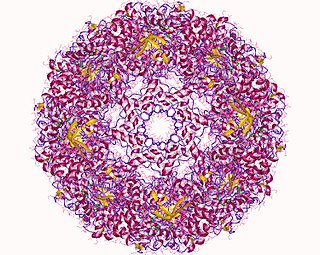
T-complex protein 1 subunit epsilon is a protein that in humans is encoded by the CCT5 gene.

Prefoldin subunit 1 is a protein that in humans is encoded by the PFDN1 gene.

Prefoldin subunit 6 is a protein that in humans is encoded by the PFDN6 gene.

T-complex protein 1 subunit eta is a protein that in humans is encoded by the CCT7 gene.

T-complex protein 1 subunit zeta is a protein that in humans is encoded by the CCT6A gene.

T-complex protein 1 subunit beta is a protein that in humans is encoded by the CCT2 gene.

Prefoldin subunit 5 is a protein that in humans is encoded by the PFDN5 gene.

T-complex protein 1 subunit gamma is a protein that in humans is encoded by the CCT3 gene.

Prefoldin subunit 2 is a protein that in humans is encoded by the PFDN2 gene.

Prefoldin subunit 4 is a protein that in humans is encoded by the PFDN4 gene.

T-complex protein 1 subunit zeta-2 is a protein that in humans is encoded by the CCT6B gene.
Proteostasis is the dynamic regulation of a balanced, functional proteome. The proteostasis network includes competing and integrated biological pathways within cells that control the biogenesis, folding, trafficking, and degradation of proteins present within and outside the cell. Loss of proteostasis is central to understanding the cause of diseases associated with excessive protein misfolding and degradation leading to loss-of-function phenotypes, as well as aggregation-associated degenerative disorders. Therapeutic restoration of proteostasis may treat or resolve these pathologies.
Chaperones, also called molecular chaperones, are proteins that assist other proteins in assuming their three-dimensional fold, which is necessary for protein function. However, the fold of a protein is sensitive to environmental conditions, such as temperature and pH, and thus chaperones are needed to keep proteins in their functional fold across various environmental conditions. Chaperones are an integral part of a cell's protein quality control network by assisting in protein folding and are ubiquitous across diverse biological taxa. Since protein folding, and therefore protein function, is susceptible to environmental conditions, chaperones could represent an important cellular aspect of biodiversity and environmental tolerance by organisms living in hazardous conditions. Chaperones also affect the evolution of proteins in general, as many proteins fundamentally require chaperones to fold or are naturally prone to misfolding, and therefore mitigates protein aggregation.

T-complex protein Ring Complex (TRiC), otherwise known as Chaperonin Containing TCP-1 (CCT), is a multiprotein complex and the chaperonin of eukaryotic cells. Like the bacterial GroEL, the TRiC complex aids in the folding of ~10% of the proteome, and actin and tubulin are some of its best known substrates. TRiC is an example of a biological machine that folds substrates within the central cavity of its barrel-like assembly using the energy from ATP hydrolysis.
















