
In immunology, an antigen (Ag) is any molecule, molecular structure, foreign particulate matter, or pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response. Antigens can be proteins, peptides, polysaccharides, lipids, or nucleic acids.

Malaria is a mosquito-borne infectious disease that affects humans and other animals. Malaria causes symptoms that typically include fever, tiredness, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. Symptoms usually begin ten to fifteen days after being bitten by an infected mosquito. If not properly treated, people may have recurrences of the disease months later. In those who have recently survived an infection, reinfection usually causes milder symptoms. This partial resistance disappears over months to years if the person has no continuing exposure to malaria.

Onchocerciasis, also known as river blindness, is a disease caused by infection with the parasitic worm Onchocerca volvulus. Symptoms include severe itching, bumps under the skin, and blindness. It is the second-most common cause of blindness due to infection, after trachoma.

Plasmodium is a genus of unicellular eukaryotes that are obligate parasites of vertebrates and insects. The life cycles of Plasmodium species involve development in a blood-feeding insect host which then injects parasites into a vertebrate host during a blood meal. Parasites grow within a vertebrate body tissue before entering the bloodstream to infect red blood cells. The ensuing destruction of host red blood cells can result in malaria. During this infection, some parasites are picked up by a blood-feeding insect, continuing the life cycle.

Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans. The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. It is responsible for around 50% of all malaria cases. P. falciparum is therefore regarded as the deadliest parasite in humans. It is also associated with the development of blood cancer and is classified as a Group 2A (probable) carcinogen.

Helminthiasis, also known as worm infection, is any macroparasitic disease of humans and other animals in which a part of the body is infected with parasitic worms, known as helminths. There are numerous species of these parasites, which are broadly classified into tapeworms, flukes, and roundworms. They often live in the gastrointestinal tract of their hosts, but they may also burrow into other organs, where they induce physiological damage.

Onchocerca volvulus is a filarial (arthropod-borne) nematode (roundworm) that causes onchocerciasis, and is the second-leading cause of blindness due to infection worldwide after trachoma. It is one of the 20 neglected tropical diseases listed by the World Health Organization, with elimination from certain countries expected by 2020.

Plasmodium vivax is a protozoal parasite and a human pathogen. This parasite is the most frequent and widely distributed cause of recurring malaria. Although it is less virulent than Plasmodium falciparum, the deadliest of the five human malaria parasites, P. vivax malaria infections can lead to severe disease and death, often due to splenomegaly. P. vivax is carried by the female Anopheles mosquito; the males do not bite.

Plasmodium malariae is a parasitic protozoan that causes malaria in humans. It is one of several species of Plasmodium parasites that infect other organisms as pathogens, also including Plasmodium falciparum and Plasmodium vivax, responsible for most malarial infection. Found worldwide, it causes a so-called "benign malaria", not nearly as dangerous as that produced by P. falciparum or P. vivax. The signs include fevers that recur at approximately three-day intervals – a quartan fever or quartan malaria – longer than the two-day (tertian) intervals of the other malarial parasites.

Plasmodium knowlesi is a parasite that causes malaria in humans and other primates. It is found throughout Southeast Asia, and is the most common cause of human malaria in Malaysia. Like other Plasmodium species, P. knowlesi has a life cycle that requires infection of both a mosquito and a warm-blooded host. While the natural warm-blooded hosts of P. knowlesi are likely various Old World monkeys, humans can be infected by P. knowlesi if they are fed upon by infected mosquitoes. P. knowlesi is a eukaryote in the phylum Apicomplexa, genus Plasmodium, and subgenus Plasmodium. It is most closely related to the human parasite Plasmodium vivax as well as other Plasmodium species that infect non-human primates.
Antigenic variation or antigenic alteration refers to the mechanism by which an infectious agent such as a protozoan, bacterium or virus alters the proteins or carbohydrates on its surface and thus avoids a host immune response, making it one of the mechanisms of antigenic escape. It is related to phase variation. Antigenic variation not only enables the pathogen to avoid the immune response in its current host, but also allows re-infection of previously infected hosts. Immunity to re-infection is based on recognition of the antigens carried by the pathogen, which are "remembered" by the acquired immune response. If the pathogen's dominant antigen can be altered, the pathogen can then evade the host's acquired immune system. Antigenic variation can occur by altering a variety of surface molecules including proteins and carbohydrates. Antigenic variation can result from gene conversion, site-specific DNA inversions, hypermutation, or recombination of sequence cassettes. The result is that even a clonal population of pathogens expresses a heterogeneous phenotype. Many of the proteins known to show antigenic or phase variation are related to virulence.
Plasmodium yoelii is a parasite of the genus Plasmodium subgenus Vinckeia. As in all Plasmodium species, P. yoelii has both vertebrate and insect hosts. The vertebrate hosts for this parasite are mammals.
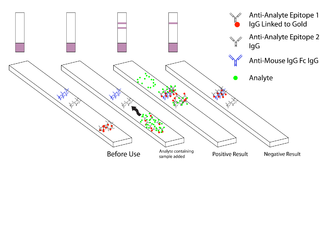
Malaria antigen detection tests are a group of commercially available rapid diagnostic tests of the rapid antigen test type that allow quick diagnosis of malaria by people who are not otherwise skilled in traditional laboratory techniques for diagnosing malaria or in situations where such equipment is not available. There are currently over 20 such tests commercially available. The first malaria antigen suitable as target for such a test was a soluble glycolytic enzyme Glutamate dehydrogenase. None of the rapid tests are currently as sensitive as a thick blood film, nor as cheap. A major drawback in the use of all current dipstick methods is that the result is essentially qualitative. In many endemic areas of tropical Africa, however, the quantitative assessment of parasitaemia is important, as a large percentage of the population will test positive in any qualitative assay.

A malaria vaccine is a vaccine that is used to prevent malaria. The only approved malaria vaccine is RTS,S, known by the brand name Mosquirix. As of April 2022, the vaccine has been given to 1 million children living in areas with moderate-to-high malaria transmission. It requires at least three doses in infants by age 2, with a fourth dose extending the protection for another 1-2 years. The vaccine reduces hospital admissions from severe malaria by around 30%.
Mosquito-borne diseases or mosquito-borne illnesses are diseases caused by bacteria, viruses or parasites transmitted by mosquitoes. Nearly 700 million people get a mosquito-borne illness each year resulting in over 725,000 deaths.
Pregnancy-associated malaria (PAM) or placental malaria is a presentation of the common illness that is particularly life-threatening to both mother and developing fetus. PAM is caused primarily by infection with Plasmodium falciparum, the most dangerous of the four species of malaria-causing parasites that infect humans. During pregnancy, a woman faces a much higher risk of contracting malaria and of associated complications. Prevention and treatment of malaria are essential components of prenatal care in areas where the parasite is endemic – tropical and subtropical geographic areas. Placental malaria has also been demonstrated to occur in animal models, including in rodent and non-human primate models.
The mainstay of malaria diagnosis has been the microscopic examination of blood, utilizing blood films. Although blood is the sample most frequently used to make a diagnosis, both saliva and urine have been investigated as alternative, less invasive specimens. More recently, modern techniques utilizing antigen tests or polymerase chain reaction have been discovered, though these are not widely implemented in malaria endemic regions. Areas that cannot afford laboratory diagnostic tests often use only a history of subjective fever as the indication to treat for malaria.

RTS,S/AS01 is a recombinant protein-based malaria vaccine. It is the only malaria vaccine approved and in wide use. As of April 2022, the vaccine has been given to 1 million children living in areas with moderate-to-high malaria transmission, with millions more doses to be provided as the vaccine's production expands. It requires at least three doses in infants by age 2, with a fourth dose extending the protection for another 1-2 years. The vaccine reduces hospital admissions from severe malaria by around 30%.

Plasmodium cynomolgi is an apicomplexan parasite that infects mosquitoes and Asian Old World monkeys. In recent years, a number of natural infections of humans have also been documented. This species has been used as a model for human Plasmodium vivax because Plasmodium cynomolgi shares the same life cycle and some important biological features with P. vivax.

Lucy Engel Graves Taliaferro was an American parasitologist and professor, notable for her research in parasitology and immunology. She and her husband, William Hay Taliaferro, worked together as research partners from 1919 to 1973. She worked as a researcher in her husband's parasitology laboratory for over 30 years at the University of Chicago, where she co-taught a parasitology course with William and achieved the rank of Assistant Professor, although she was never paid. After retiring from the University of Chicago in 1960, the Talioferros researched the effects of ionizing radiation on the immune response at Argonne National Laboratory and coauthored Radiation and Immune Mechanisms (1964).
















