
Trastuzumab, sold under the brand name Herceptin among others, is a monoclonal antibody used to treat breast cancer and stomach cancer. It is specifically used for cancer that is HER2 receptor positive. It may be used by itself or together with other chemotherapy medication. Trastuzumab is given by slow injection into a vein and injection just under the skin.
A cancer vaccine, or oncovaccine, is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology (immuno-oncology) and a growing subspecialty of oncology.
An oncoantigen is a surface or soluble tumor antigen that supports tumor growth. A major problem of cancer immunotherapy is the selection of tumor cell variants that escape immune recognition. The notion of oncoantigen was set forth in the context of cancer immunoprevention to define a class of persistent tumor antigens not prone to escape from immune recognition.
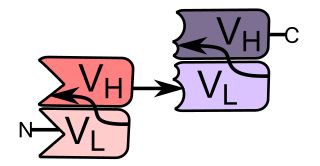
Bi-specific T-cell engagers (BiTEs) are a class of artificial bispecific monoclonal antibodies that are investigated for use as anti-cancer drugs. They direct a host's immune system, more specifically the T cells' cytotoxic activity, against cancer cells. BiTE is a registered trademark of Micromet AG.
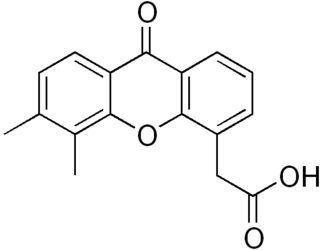
Vadimezan is a tumor-vascular disrupting agent (tumor-VDA) that attacks the blood supply of a cancerous tumor to cause tumor regression.

Phosphoinositide 3-kinase inhibitors are a class of medical drugs that are mainly used to treat advanced cancers. They function by inhibiting one or more of the phosphoinositide 3-kinase (PI3K) enzymes, which are part of the PI3K/AKT/mTOR pathway. This signal pathway regulates cellular functions such as growth and survival. It is strictly regulated in healthy cells, but is always active in many cancer cells, allowing the cancer cells to better survive and multiply. PI3K inhibitors block the PI3K/AKT/mTOR pathway and thus slow down cancer growth. They are examples of a targeted therapy. While PI3K inhibitors are an effective treatment, they can have very severe side effects and are therefore only used if other treatments have failed or are not suitable.

ALK inhibitors are anti-cancer drugs that act on tumours with variations of anaplastic lymphoma kinase (ALK) such as an EML4-ALK translocation. They fall under the category of tyrosine kinase inhibitors, which work by inhibiting proteins involved in the abnormal growth of tumour cells. All the current approved ALK inhibitors function by binding to the ATP pocket of the abnormal ALK protein, blocking its access to energy and deactivating it. A majority of ALK-rearranged NSCLC harbour the EML4-ALK fusion, although as of 2020, over 92 fusion partners have been discovered in ALK+ NSCLC. For each fusion partner, there can be several fusion variants depending on the position the two genes were fused at, and this may have implications on the response of the tumour and prognosis of the patient.
Neuvenge, Lapuleucel-T, is a therapeutic cancer vaccine (TCV) in development by Dendreon (DNDN). It uses the "immunotherapy platform approach" first successfully demonstrated on the U.S. Food and Drug Administration (FDA)-approved TCV Provenge. It was first tested on breast cancer patients with tumors expressing HER2/neu, and is now scheduled to be tested on bladder cancer patients.

Cellectis is a French biopharmaceutical company. It develops genome-edited chimeric antigen receptor T-cell technologies for cancer immunotherapy. It has offices in Paris, New York City, and Raleigh, North Carolina.
Kite Pharma is an American biotechnology company that develops cancer immunotherapy products with a primary focus on genetically engineered autologous CAR T cell therapy - a cell-based therapy which relies on chimeric antigen receptors and T cells. Founded in 2009, and based in Santa Monica, California, it was acquired by Gilead Sciences in 2017.

Immutep Ltd is a biotechnology company working primarily in the field of cancer immunotherapy using the LAG3 immune control mechanism. The company was originally built on CVac, a therapeutic cancer vaccine. In late 2014 the privately held French immunotherapy company Immutep SA was purchased by Prima Biotech.

Juno Therapeutics Inc was an American biopharmaceutical company founded in 2013 through a collaboration of the Fred Hutchinson Cancer Research Center, Memorial Sloan-Kettering Cancer Center and pediatrics partner Seattle Children's Research Institute. The company was launched with an initial investment of $120 million, with a remit to develop a pipeline of cancer immunotherapy drugs. The company raised $300 million through private funding and a further $265 million through their IPO.

SOTIO Biotech is a Czech biotechnology company focused on clinical-stage research and development of innovative medicines for cancer with operations in Europe, North America, and Asia. The company has clinical programs which include a superagonist of the immuno-oncology target IL-15, a new generation of potent and stable antibody-drug conjugates (ADCs), proprietary technology designed to improve on the efficacy of CAR T therapies and a platform to streamline and enhance personalized cell therapies.
Imugene Ltd is a clinical stage immuno-oncology company developing a range of new and novel immunotherapies that seek to activate the immune system of cancer patients to treat and eradicate tumours. Imugene's unique platform technologies seeks to harness the body's immune system against tumours, potentially achieving a similar or greater effect than synthetically manufactured monoclonal antibody and other immunotherapies.
Paul A. Hopper is an Australian bioentrepreneur who has been associated since 2003 with a number of biotechnology companies, most of them publicly traded.
Viralytics Ltd is an Australian biotechnology company working in the field of oncolytic viruses, that is, viruses that preferentially infect and kill cancer cells. The company's oncolytic virus product, called Cavatak, is currently in clinical trials in metastatic melanoma and other cancers. The drug was granted Orphan Drug status in advanced melanoma in December 2005.
Said Sebti (Arabic: سيد سبتي, (first name is an American cancer researcher who is Professor and Chairman of the Department of Drug Discovery at the H. Lee Moffitt Cancer Center & Research Institute in Tampa, Fl. Sebti is noted for his work to rehabilitate the 'failed' cancer drug Triciribine, now under development at the pharmaceutical company Prescient Therapeutics. Sebti is currently Chief Scientific Officer at Prescient Therapeutics.
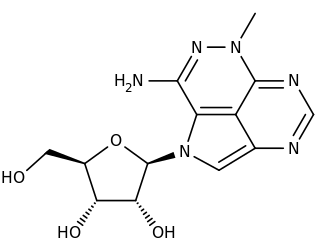
Triciribine is a cancer drug which was first synthesized in the 1970s and studied clinically in the 1980s and 1990s without success. Following the discovery in the early 2000s that the drug would be effective against tumours with hyperactivated Akt, it is now again under consideration in a variety of cancers. As PTX-200, the drug is currently in two early stage clinical trials in breast cancer and ovarian cancer being conducted by the small molecule drug development company Prescient Therapeutics.
GEMoaB is a biopharmaceutical company based in Dresden/Germany. The company has a broad pipeline of next generation immunotherapy product candidates for the treatment of advanced blood cancers and solid tumours in pre-clinical and clinical development. The company was founded in 2011 by the two university professors Gerhard Ehninger and Michael Bachmann. It was acquired by the new company AvenCell Therapeutics in 2021.










