Related Research Articles

Lipid-anchored proteins are proteins located on the surface of the cell membrane that are covalently attached to lipids embedded within the cell membrane. These proteins insert and assume a place in the bilayer structure of the membrane alongside the similar fatty acid tails. The lipid-anchored protein can be located on either side of the cell membrane. Thus, the lipid serves to anchor the protein to the cell membrane. They are a type of proteolipids.
The C-terminus is the end of an amino acid chain, terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus.
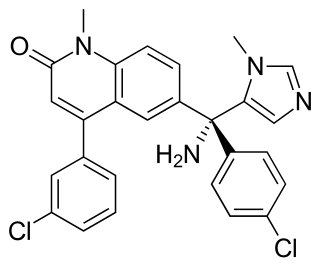
The farnesyltransferase inhibitors (FTIs) are a class of experimental cancer drugs that target protein farnesyltransferase with the downstream effect of preventing the proper functioning of the Ras (protein), which is commonly abnormally active in cancer.

Prenylation is the addition of hydrophobic molecules to a protein or a biomolecule. It is usually assumed that prenyl groups (3-methylbut-2-en-1-yl) facilitate attachment to cell membranes, similar to lipid anchors like the GPI anchor, though direct evidence of this has not been observed. Prenyl groups have been shown to be important for protein–protein binding through specialized prenyl-binding domains.
Farnesyltransferase is one of the three enzymes in the prenyltransferase group. Farnesyltransferase (FTase) adds a 15-carbon isoprenoid called a farnesyl group to proteins bearing a CaaX motif: a four-amino acid sequence at the carboxyl terminus of a protein. Farnesyltransferase's targets include members of the Ras superfamily of small GTP-binding proteins critical to cell cycle progression. For this reason, several FTase inhibitors are undergoing testing as anti-cancer agents. FTase inhibitors have shown efficacy as anti-parasitic agents, as well. FTase is also believed to play an important role in development of progeria and various forms of cancers.

Rab geranylgeranyltransferase also known as (protein) geranylgeranyltransferase II is one of the three prenyltransferases. It transfers (usually) two geranylgeranyl groups to the cystein(s) at the C-terminus of Rab proteins.
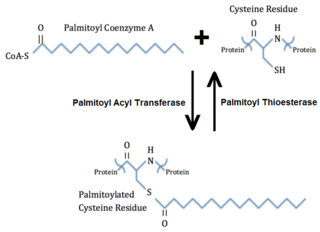
Palmitoylation is the covalent attachment of fatty acids, such as palmitic acid, to cysteine (S-palmitoylation) and less frequently to serine and threonine (O-palmitoylation) residues of proteins, which are typically membrane proteins. The precise function of palmitoylation depends on the particular protein being considered. Palmitoylation enhances the hydrophobicity of proteins and contributes to their membrane association. Palmitoylation also appears to play a significant role in subcellular trafficking of proteins between membrane compartments, as well as in modulating protein–protein interactions. In contrast to prenylation and myristoylation, palmitoylation is usually reversible (because the bond between palmitic acid and protein is often a thioester bond). The reverse reaction in mammalian cells is catalyzed by acyl-protein thioesterases (APTs) in the cytosol and palmitoyl protein thioesterases in lysosomes. Because palmitoylation is a dynamic, post-translational process, it is believed to be employed by the cell to alter the subcellular localization, protein–protein interactions, or binding capacities of a protein.

The PT-barrel, is a novel protein fold that was discovered in the crystal structure of the prenyltransferase, Orf2 from Streptomyces sp. strain CL190.
Geranylgeranylation is a form of prenylation, which is a post-translational modification of proteins that involves the attachment of one or two 20-carbon lipophilic geranylgeranyl isoprene units from geranylgeranyl diphosphate to one or two cysteine residue(s) at the C-terminus of specific proteins. Prenylation is thought to function, at least in part, as a membrane anchor for proteins.

Prenyltransferases (PTs) are a class of enzymes that transfer allylic prenyl groups to acceptor molecules. Prenyl transferases commonly refer to isoprenyl diphosphate syntheses (IPPSs). Prenyltransferases are a functional category and include several enzyme groups that are evolutionarily independent.
In enzymology, a geranyltranstransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphoglycerol geranylgeranyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a protein geranylgeranyltransferase type I is an enzyme that catalyzes the chemical reaction

Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha is an enzyme that in humans is encoded by the FNTA gene.

Protein farnesyltransferase subunit beta is an enzyme that in humans is encoded by the FNTB gene.

Geranylgeranyl pyrophosphate synthase is an enzyme that in humans is encoded by the GGPS1 gene.

Nuclear prelamin A recognition factor, also known as NARF, is a protein which in humans is encoded by the NARF gene.
In molecular biology the DHHC domain is a protein domain that acts as an enzyme, which adds a palmitoyl chemical group to proteins in order to anchor them to cell membranes. The DHHC domain was discovered in 1999 and named after a conserved sequence motif found in its protein sequence. Roth and colleagues showed that the yeast Akr1p protein could palmitoylate Yck2p in vitro and inferred that the DHHC domain defined a large family of palmitoyltransferases. In mammals twenty three members of this family have been identified and their substrate specificities investigated. Some members of the family such as ZDHHC3 and ZDHHC7 enhance palmitoylation of proteins such as PSD-95, SNAP-25, GAP43, Gαs. Others such as ZDHHC9 showed specificity only toward the H-Ras protein. However, a recent study questions the involvement of classical enzyme-substrate recognition and specificity in the palmitoylation reaction. Several members of the family have been implicated in human diseases.

Protein geranylgeranyltransferase type I subunit beta is a protein that in humans is encoded by the PGGT1B gene.

Patrick J. (Pat) Casey is a biochemist and molecular pharmacologist and is a James B. Duke Professor of Pharmacology and Cancer Biology at Duke University School of Medicine. In 2005, he relocated to Singapore to help found the Duke-NUS Medical School Singapore, where he continues to serve as its Senior Vice Dean of Research.
References
- 1 2 Lane KT, Beese LS (April 2006). "Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I". J. Lipid Res. 47 (4): 681–99. doi: 10.1194/jlr.R600002-JLR200 . PMID 16477080.
- ↑ Reid TS, Terry KL, Casey PJ, Beese LS (October 2004). "Crystallographic analysis of CaaX prenyltransferases complexed with substrates defines rules of protein substrate selectivity". J. Mol. Biol. 343 (2): 417–33. doi:10.1016/j.jmb.2004.08.056. PMID 15451670.
- ↑ Long SB, Casey PJ, Beese LS (October 2002). "Reaction path of protein farnesyltransferase at atomic resolution". Nature. 419 (6907): 645–50. Bibcode:2002Natur.419..645L. doi:10.1038/nature00986. PMID 12374986. S2CID 4412580.